Overview
Regulatory affairs in pharmaceuticals represent a pivotal discipline, ensuring compliance with the laws and regulations that govern the development, testing, manufacturing, and marketing of medications. This compliance is essential for safeguarding public health.
Compliance specialists play a crucial role in overseeing the entire lifecycle of pharmaceutical products, ensuring they meet rigorous safety and efficacy standards. This oversight is vital for maintaining trust among stakeholders and facilitating timely access to innovative therapies.
The expertise of these specialists not only protects consumers but also enhances the credibility of the pharmaceutical industry.
Introduction
The pharmaceutical landscape represents a complex web of innovation, regulation, and public health, where the stakes could not be higher. Regulatory affairs serve as the backbone of this industry, ensuring that every medication brought to market meets stringent safety and efficacy standards. As this field evolves—driven by technological advancements and changing regulations—understanding the nuances of regulatory affairs becomes crucial for anyone involved in drug development. Regulatory professionals face significant challenges in maintaining compliance while fostering innovation, influencing the future dynamics of pharmaceuticals. What implications do these challenges hold for the industry as a whole?
Define Regulatory Affairs in Pharmaceuticals
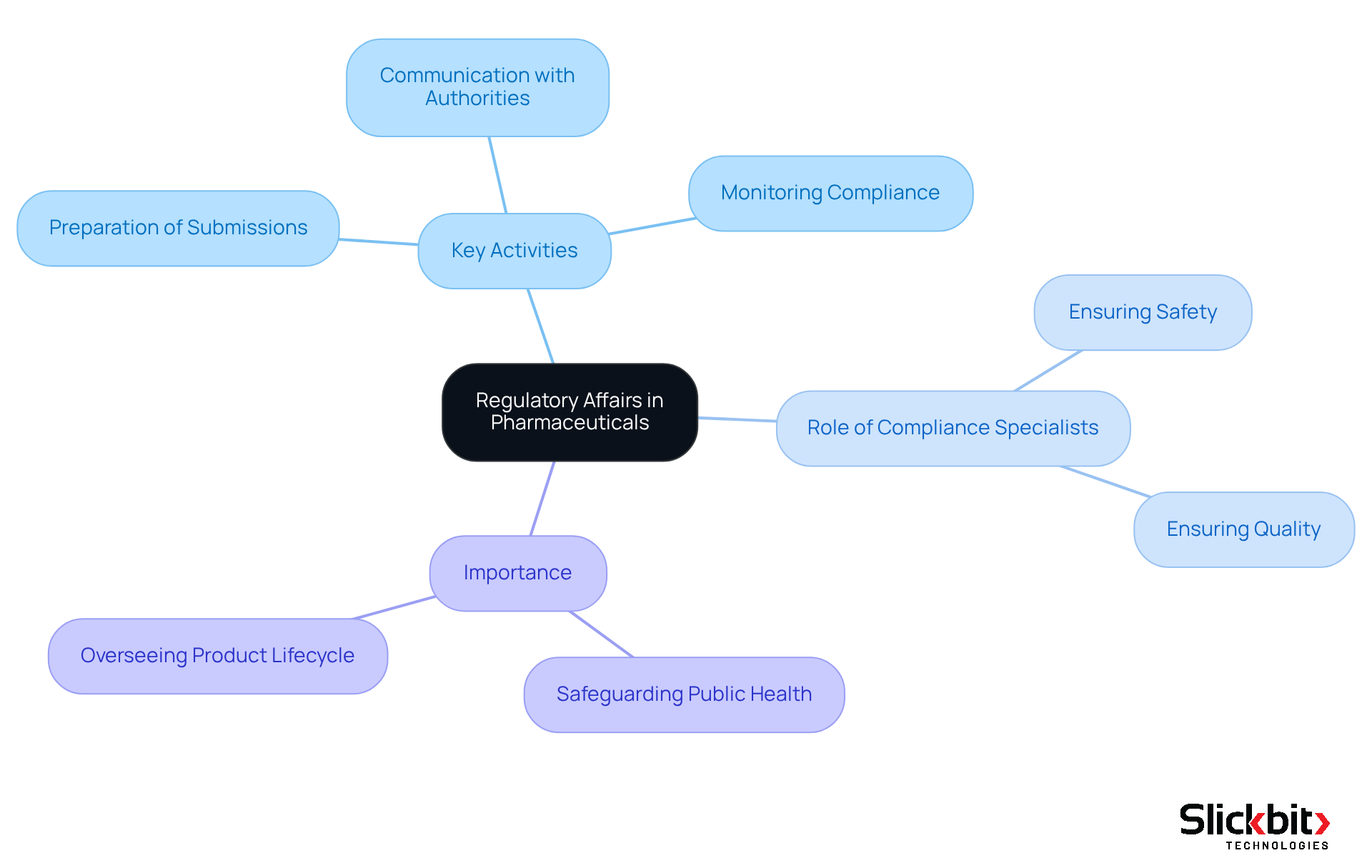
What is regulatory affairs in pharma is a critical discipline in the drug industry that ensures adherence to laws and regulations governing the development, testing, manufacturing, and marketing of medications. This area encompasses a wide range of activities, including:
- The preparation of submissions
- Communication with authorities
- Monitoring compliance with relevant regulations
Compliance specialists act as a vital link between drug manufacturers and oversight bodies, ensuring that products are safe, effective, and of high quality. Their work is indispensable in safeguarding public health, as they oversee the entire lifecycle of pharmaceutical products from conception to market release. Consequently, understanding what is regulatory affairs in pharma is essential for all stakeholders involved in the pharmaceutical industry.
Trace the History and Evolution of Regulatory Affairs
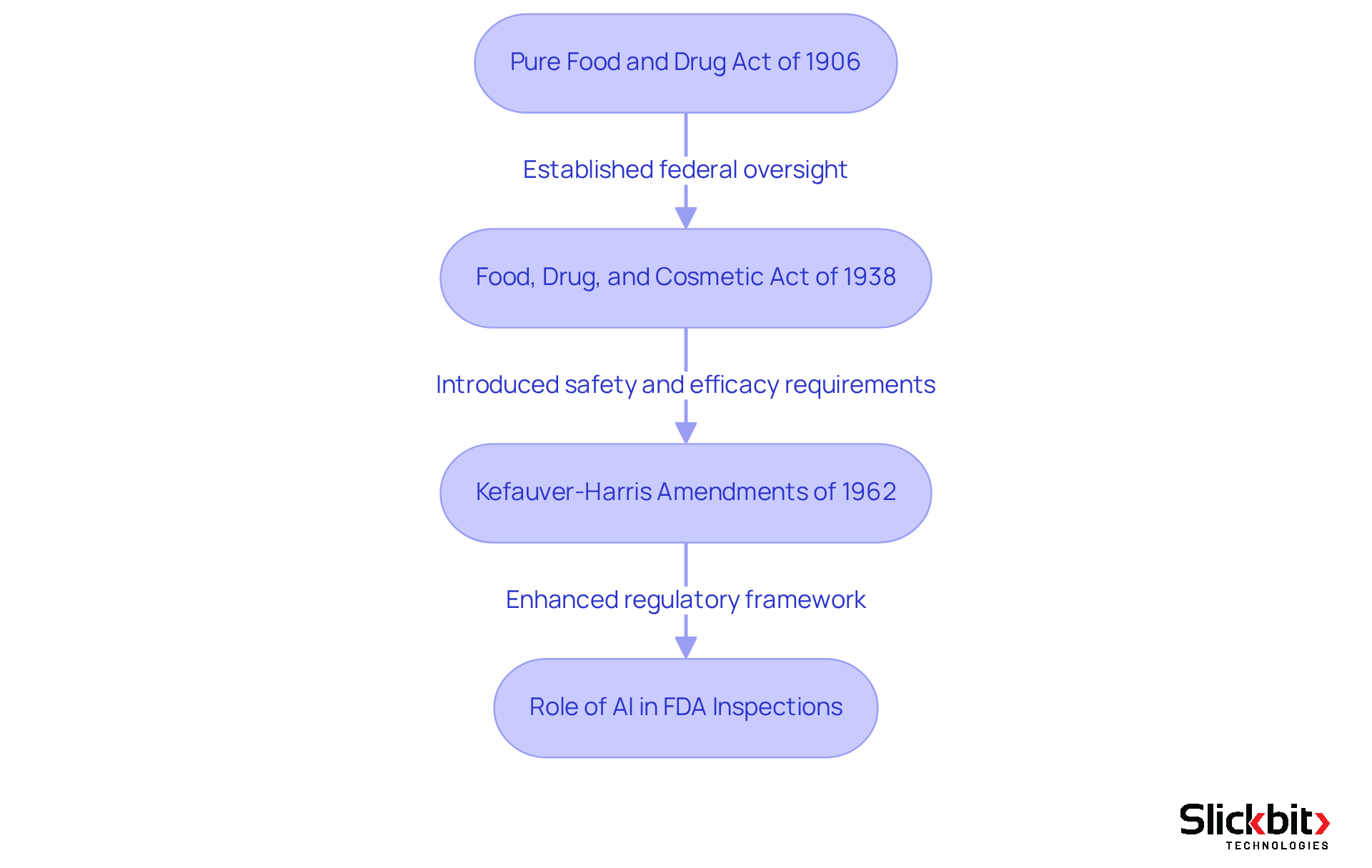
The development of regulatory affairs in the biopharmaceutical sector commenced in the early 20th century, marked by pivotal milestones that significantly influenced safety and efficacy. The Pure Food and Drug Act of 1906 emerged as a revolutionary piece of legislation, establishing the foundation for federal oversight of food and medications in the United States. This act sought to eradicate misbranding and adulteration, ensuring that consumers received safe products. In the wake of this, the Food, Drug, and Cosmetic Act of 1938 introduced more stringent requirements for drug approval, necessitating that all new drugs be proven safe prior to marketing. This legislation was a direct response to public health crises, notably the Elixir Sulfanilamide disaster, which resulted in over 100 fatalities and underscored the urgent need for reform in oversight.
As the decades unfolded, the regulatory framework continued to evolve in response to emerging scientific advancements and public health demands. The establishment of the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) formalized the regulatory process, creating a structured approach to medication evaluation and approval. The Kefauver-Harris Amendments of 1962 further strengthened regulations by requiring that medications demonstrate effectiveness in addition to safety, reflecting an increasing emphasis on consumer protection.
Over the years, the evolution of medication regulations has been influenced by various factors, including the rise of direct-to-consumer advertising, which currently accounts for nearly 40 percent of overall promotional spending in the industry, alongside the growing complexity of marketing in this sector. The FDA's role has expanded to encompass not only drug safety but also the promotion of public health through effective communication and education regarding medications. Today, understanding what is regulatory affairs in pharma is essential, as it represents a complex and multifaceted discipline crucial for ensuring that pharmaceutical products adhere to the highest standards of safety and efficacy while navigating the challenges of a rapidly evolving healthcare landscape. Furthermore, the integration of AI technologies in FDA inspections is revolutionizing adherence practices, facilitating more effective detection of systemic risks and recurring violations, thereby enhancing the overall oversight structure.
Identify Key Responsibilities and Functions in Regulatory Affairs
Key responsibilities in what is regulatory affairs in pharma encompass the preparation and submission of official documents, conducting intelligence to remain abreast of evolving laws, and ensuring strict adherence to Good Manufacturing Practices (GMP). Professionals in compliance affairs actively engage in risk evaluation, oversee audits, and communicate with governing bodies to ensure they understand what is regulatory affairs in pharma throughout the drug development process. They play a pivotal role in:
- Clinical trial applications
- Post-marketing surveillance
- The creation of labeling and promotional materials
These are all aspects of what is regulatory affairs in pharma, ensuring that all communications align with compliance standards.
Furthermore, by leveraging AI tools such as Trend 483, the efficiency of these processes can be significantly enhanced. These tools are instrumental in detecting trends in systemic risks and regulatory challenges arising from FDA 483s, providing deeper insights that facilitate informed regulatory decision-making.

Explain the Importance of Compliance in Drug Development
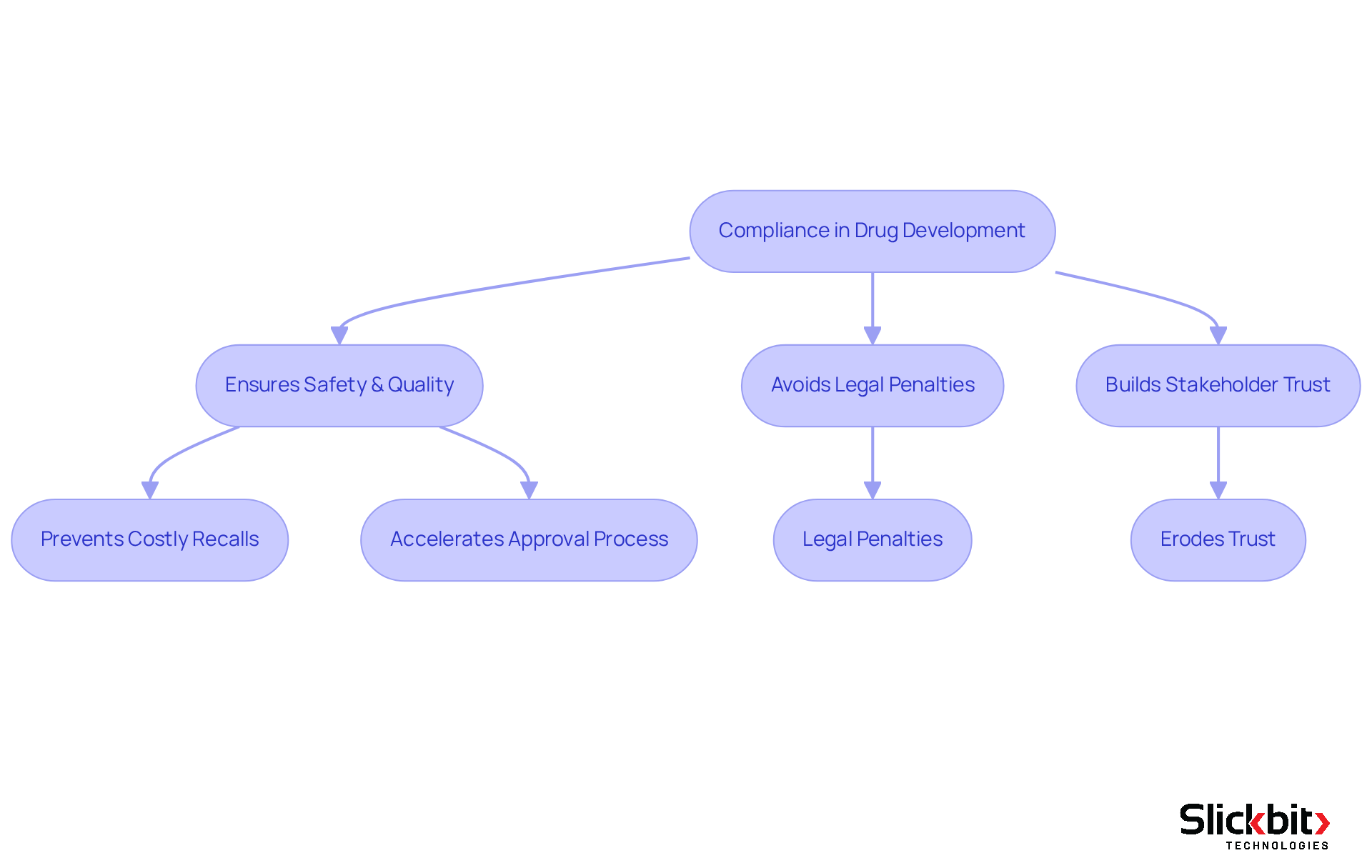
Compliance in drug development is essential for ensuring that pharmaceutical products are safe, effective, and of the highest quality. Non-compliance can lead to severe consequences, including costly recalls, legal penalties, and significant reputational damage. A recent FDA Warning Letter emphasized significant Good Manufacturing Practice (CGMP) violations, underscoring the necessity for strict adherence to regulatory standards. Such failures not only jeopardize public health but also erode trust among stakeholders, including healthcare providers and patients, who rely on the assurance that products meet rigorous safety standards.
Furthermore, adherence plays a crucial role in enabling smoother interactions with oversight bodies, greatly accelerating the approval process. Statistics indicate that adherence issues can extend drug approval timelines by several months, delaying patient access to innovative therapies. In a sector where prompt access to medications can be a matter of life and death, understanding what is regulatory affairs in pharma is essential for ensuring adherence. By prioritizing compliance, pharmaceutical companies can enhance their operational efficiency and ensure that they bring safe and effective products to market without unnecessary delays.
Conclusion
Regulatory affairs in the pharmaceutical sector serve as a cornerstone for ensuring that medications are developed, tested, and marketed in strict compliance with established laws and regulations. This discipline not only safeguards public health but also fosters trust among stakeholders by ensuring that pharmaceutical products adhere to the highest standards of safety and efficacy. Understanding the intricacies of regulatory affairs is essential for all participants in the drug development process, as it directly influences the success of bringing innovative therapies to market.
The article explores the historical evolution of regulatory affairs, highlighting pivotal legislation that has shaped the industry, including:
- The Pure Food and Drug Act
- The establishment of key regulatory bodies such as the FDA and EMA
It emphasizes the critical responsibilities of compliance specialists, which encompass:
- The preparation of submissions
- Monitoring compliance
- Upholding Good Manufacturing Practices
Furthermore, it underscores how advancements, particularly the integration of AI, are enhancing the efficiency of regulatory processes, thereby addressing systemic risks more effectively.
In light of the complexities surrounding drug development, prioritizing compliance transcends mere regulatory necessity; it is a moral imperative. Non-compliance can lead to dire consequences, ranging from costly recalls to compromised public trust. As the pharmaceutical landscape continues to evolve, the importance of regulatory affairs will only intensify, making it imperative for industry professionals to remain informed and proactive. Embracing these insights can lead to improved operational efficiency and ultimately better health outcomes for patients worldwide.
Frequently Asked Questions
What is regulatory affairs in pharmaceuticals?
Regulatory affairs in pharmaceuticals is a critical discipline that ensures adherence to laws and regulations governing the development, testing, manufacturing, and marketing of medications.
What activities are involved in regulatory affairs?
Regulatory affairs encompasses activities such as the preparation of submissions, communication with authorities, and monitoring compliance with relevant regulations.
What is the role of compliance specialists in regulatory affairs?
Compliance specialists act as a vital link between drug manufacturers and oversight bodies, ensuring that products are safe, effective, and of high quality.
Why is regulatory affairs important in the pharmaceutical industry?
Regulatory affairs is essential for safeguarding public health, as it oversees the entire lifecycle of pharmaceutical products from conception to market release.
Who should understand regulatory affairs in pharmaceuticals?
Understanding regulatory affairs is essential for all stakeholders involved in the pharmaceutical industry.




