Overview
The Company Core Data Sheet (CCDS) serves as a pivotal document within the pharmaceutical industry, consolidating vital information about medications to ensure regulatory compliance and safeguard patient safety. This article underscores its essential role in providing standardized drug labeling, effectively managing product information, and empowering healthcare professionals to make informed decisions. Consequently, the CCDS significantly enhances drug safety and operational efficiency in pharmaceutical operations, establishing itself as an indispensable resource for industry stakeholders.
Introduction
The pharmaceutical industry operates within a complex web of regulations and safety standards, where the accuracy of medication information can significantly impact patient outcomes. At the heart of this framework lies the Company Core Data Sheet (CCDS), a crucial document that consolidates essential details about a drug's characteristics, applications, and safety. As the industry evolves, the challenge of maintaining compliance intensifies, necessitating that healthcare professionals have access to the most current information about medications. Consequently, the CCDS plays a vital role in navigating these challenges, contributing to safer prescribing practices in an ever-changing regulatory landscape.
Define CCDS: Core Concept and Purpose
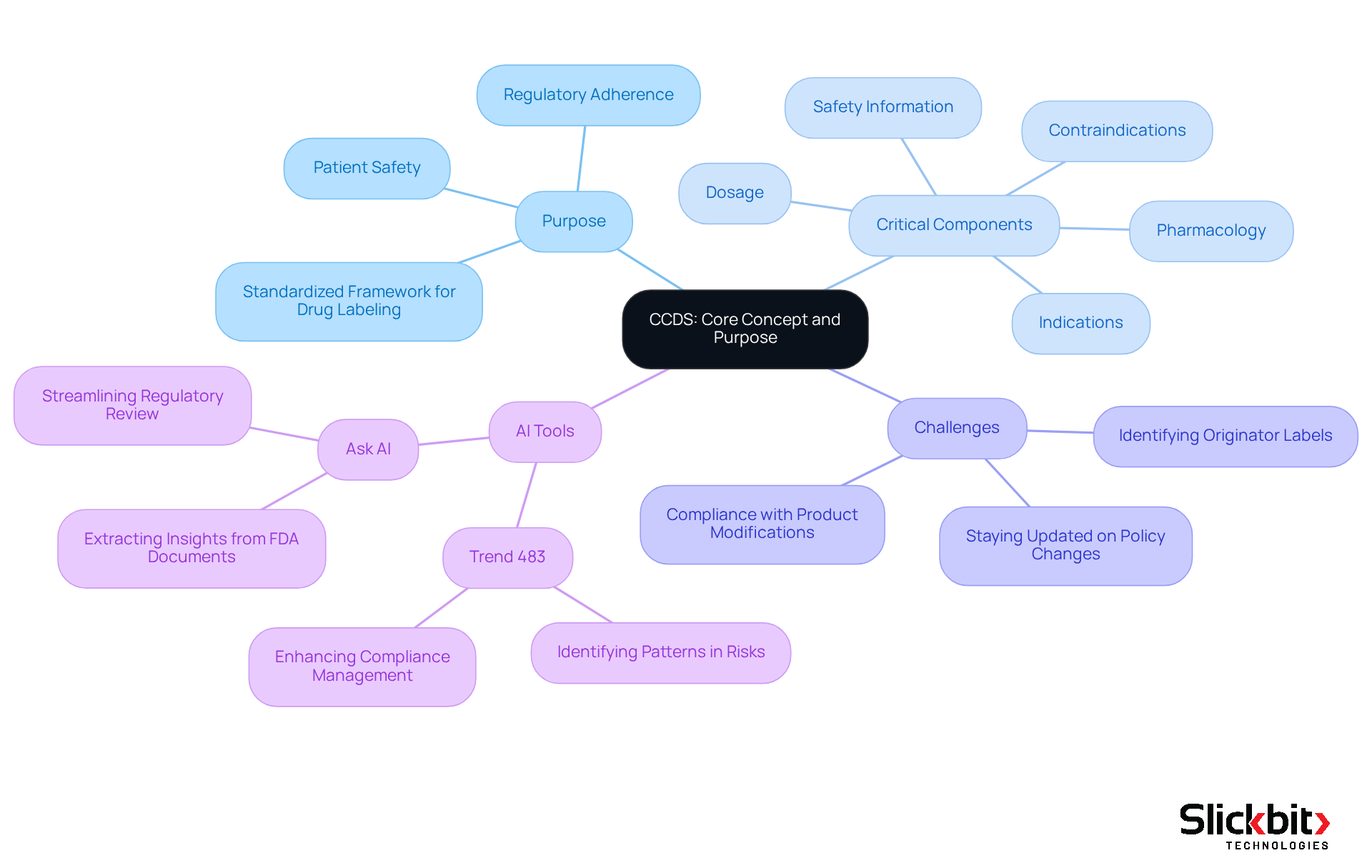
What is ccds? The Company Core Data Sheet stands as a pivotal document within the pharmaceutical industry, serving as a comprehensive reference that consolidates essential information about a medication's characteristics, applications, and safety. Centrally maintained by the Marketing Authorization Holder (MAH), this document embodies the company's official position on the product, guaranteeing that all stakeholders have access to consistent and accurate information. It is designed to provide a standardized framework for drug labeling and compliance submissions, which is vital for understanding what is ccds in relation to regulatory adherence and patient safety.
This document encompasses critical components such as:
- Indications
- Contraindications
- Dosage
- Pharmacology
- Safety information
Rendering it an invaluable resource for healthcare professionals and regulatory authorities alike. Furthermore, the system aids in monitoring product performance and facilitating necessary enhancements, thereby improving compliance efficiency. However, managing this system presents challenges, including the need to stay updated on policy changes and ensuring compliance with product modifications.
AI tools, such as Trend 483, can significantly streamline this process by identifying patterns in systemic risks and recurrent violations, thus enhancing the management of updates and bolstering compliance efforts. Non-compliance with established standards can lead to penalties and product recalls, underscoring the critical importance of adhering to guidelines. The historical evolution of this system highlights its essential role in clinical research, reflecting a trend towards greater transparency and rigor in pharmaceutical development.
Trace the Evolution of CCDS in Life Sciences
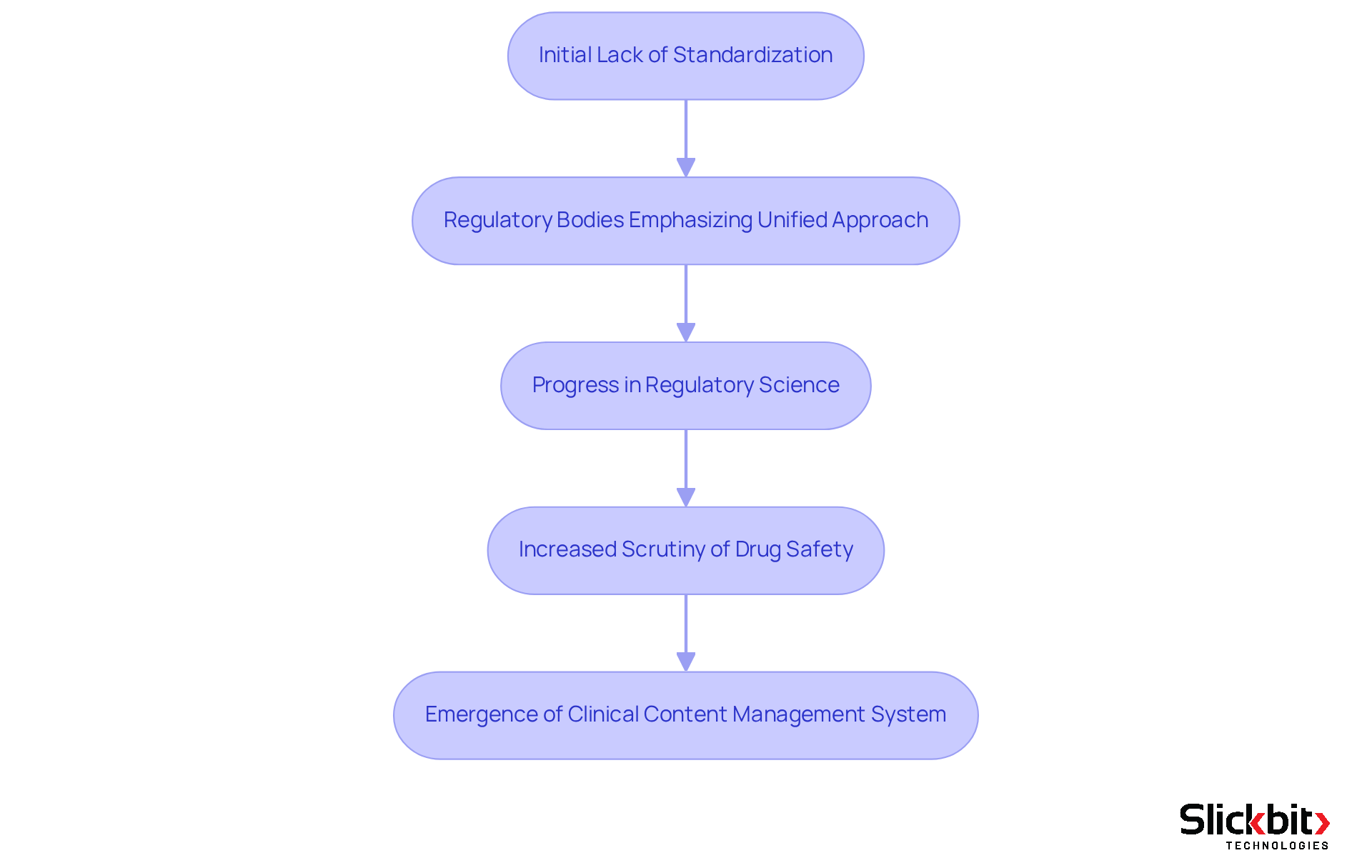
The development of the Company Core Data Sheet (CCDS) highlights what is CCDS and signifies a pivotal advancement aimed at enhancing patient protection and ensuring regulatory compliance. Initially, the absence of standardization in medication labeling led to discrepancies that posed risks to patient safety. In response, regulatory bodies have increasingly underscored the importance of a unified approach to medication information. This evolution has been driven by progress in regulatory science, intensified scrutiny of drug safety, and the urgent need for harmonization across international markets. Consequently, the Clinical Content Management System has emerged as an essential element in the lifecycle of pharmaceutical products, influencing processes from clinical trials to post-marketing surveillance. The historical progression of this system underscores a broader dedication to providing healthcare professionals with accurate and comprehensive medication information, ultimately promoting safer prescribing practices and informed decision-making.
Examine Key Components of CCDS
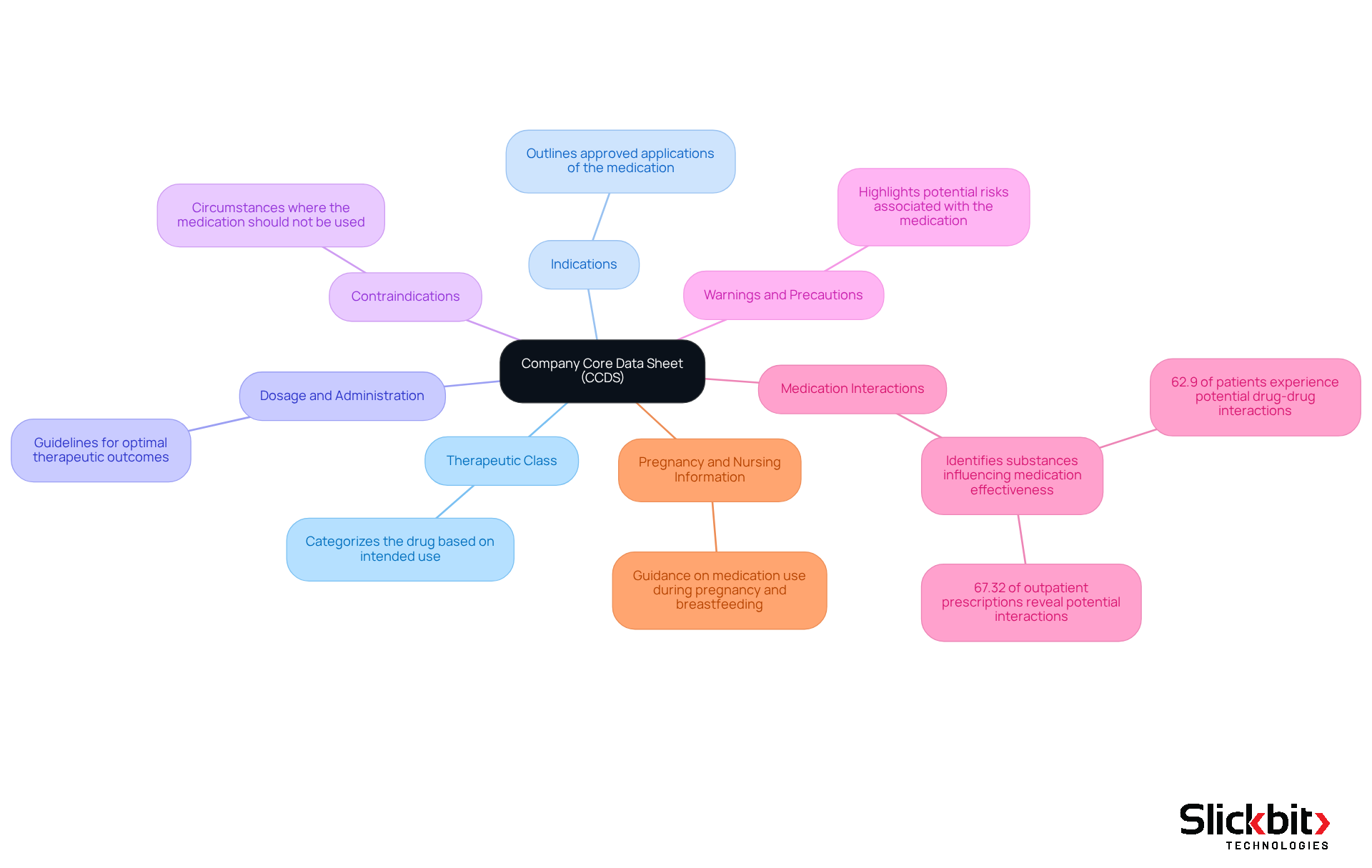
A typical Company Core Data Sheet (CCDS) encompasses several essential components crucial for drug safety and efficacy:
- Therapeutic Class: This categorizes the drug based on its intended use, providing a framework for understanding its application in treatment protocols.
- Indications: This section outlines the approved applications of the medication, ensuring that healthcare providers are aware of the specific conditions it is intended to address.
- Dosage and Administration: Guidelines on how the medication should be administered are outlined here, which is vital for optimizing therapeutic outcomes and minimizing risks.
- Contraindications: This outlines circumstances in which the medication should not be utilized, aiding in the avoidance of adverse effects and ensuring patient well-being.
- Warnings and Precautions: Potential risks associated with the medication are highlighted, allowing healthcare providers to make informed decisions regarding patient care.
- Medication Interactions: This identifies other substances that may influence the medication's effectiveness or security. Studies indicate that 62.9% of patients experience potential medication-medication interactions in clinical settings, and 67.32% of analyzed outpatient prescriptions revealed potential medication-medication interactions.
- Pregnancy and Nursing Information: Guidance on the medication's use during pregnancy and breastfeeding is provided, which is critical for protecting vulnerable populations.
Each of these components plays a vital role in ensuring that healthcare providers have the necessary information to make informed decisions about patient care. The centralized database functions as a repository of information for creating and updating pharmaceutical product labels, significantly enhancing patient safety by informing healthcare professionals about drug interactions, dosing, and potential adverse effects. By maintaining a precise and thorough document—an obligation of the Marketing Authorization Holder (MAH)—pharmaceutical firms can enhance regulatory adherence and minimize the chance of product recalls. This ultimately results in improved healthcare outcomes. The document serves as a dynamic resource that offers current information on specific products, and its creation entails a cooperative process involving drafting, reviewing, and securing approvals from various departments.
Highlight the Importance of CCDS in Pharmaceutical Operations
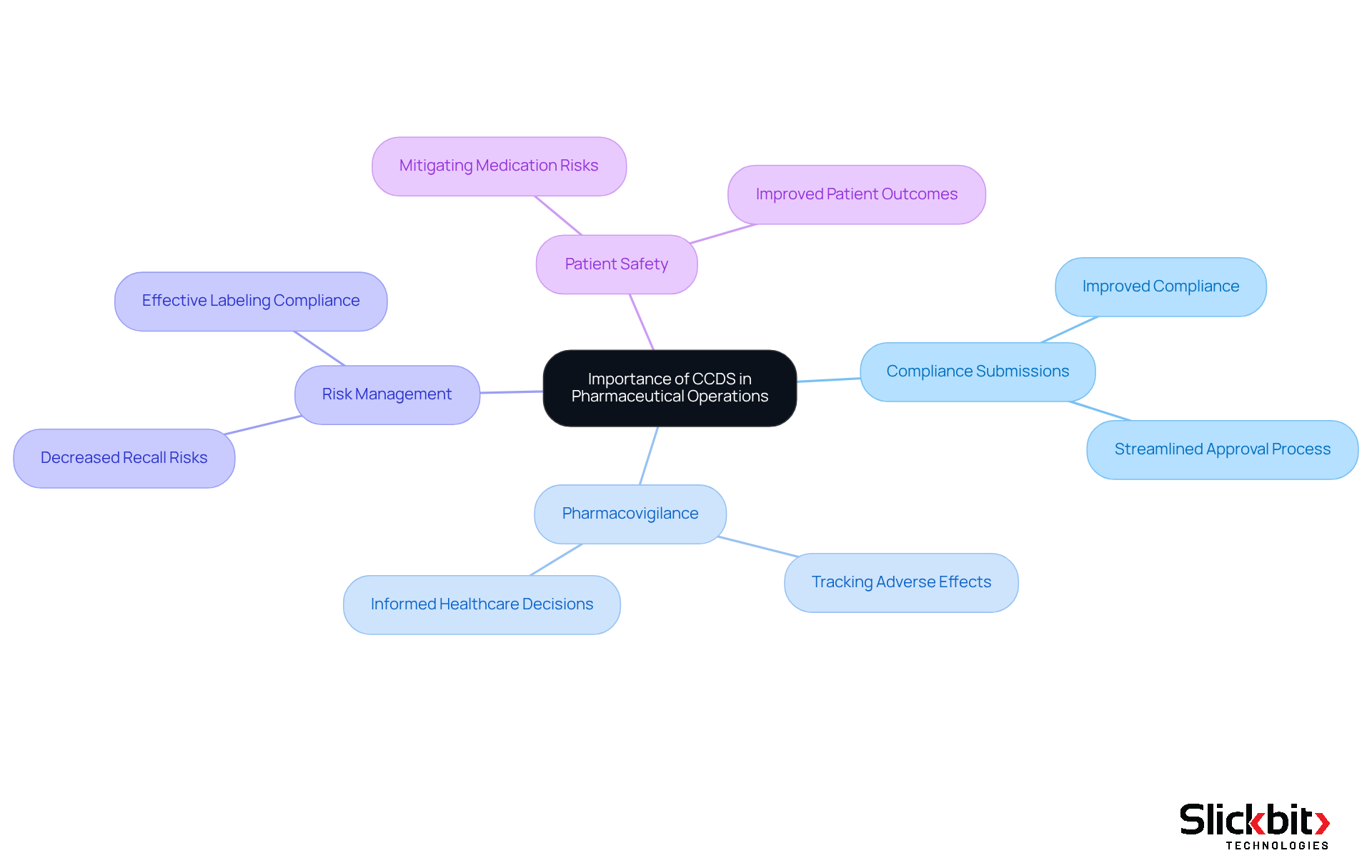
Understanding what is CCDS is pivotal in pharmaceutical operations for several reasons. Primarily, it serves as the foundation for compliance submissions, ensuring that all necessary information is presented consistently. This consistency is vital for maintaining adherence to standards and streamlining the approval process for new drugs. Companies employing such systems have reported improved compliance and decreased recall risks, demonstrating its effectiveness in regulatory contexts.
Furthermore, the centralized database plays a crucial role in pharmacovigilance by offering a unified reference for tracking adverse effects and ensuring that risk information is conveyed efficiently. By combining essential information, the system enhances healthcare professionals' capacity to make informed choices, ultimately improving patient well-being and outcomes. For instance, potential drug-drug interactions were identified in over 67% of analyzed outpatient prescriptions, underscoring the importance of thorough documentation in mitigating risks.
In addition, the system assists the marketing authorization holder (MAH) in managing product information across different regions, ensuring that labeling complies with local regulations. This alignment is crucial for effective risk management and aids healthcare professionals in making safe prescribing decisions. What is CCDS? It is a structured content that not only enhances regulatory compliance but also promotes patient safety, making it an indispensable tool in the pharmaceutical industry.
Conclusion
The Company Core Data Sheet (CCDS) stands as a cornerstone in pharmaceutical operations, providing a comprehensive framework that guarantees the accuracy and consistency of vital medication information. By centralizing essential details about drug characteristics, applications, and safety, the CCDS not only aids in regulatory compliance but also enhances patient safety across the healthcare spectrum.
This article has explored the pivotal role of the CCDS, highlighting its critical components such as:
- Indications
- Contraindications
- Dosage
- Safety information
The evolution of this document reflects a broader commitment to standardization and transparency within the pharmaceutical industry, addressing historical discrepancies that could jeopardize patient care. Furthermore, the integration of advanced AI tools has streamlined compliance processes, thereby reducing the risk of non-compliance and its associated penalties.
In light of these insights, the importance of the CCDS cannot be overstated. As the pharmaceutical landscape continues to evolve, embracing structured content like the CCDS is essential for ensuring safe prescribing practices and informed decision-making. Stakeholders across the industry are encouraged to prioritize the implementation and management of the CCDS to foster a culture of safety and compliance, ultimately leading to improved healthcare outcomes and enhanced patient well-being.
Frequently Asked Questions
What is CCDS?
CCDS stands for Company Core Data Sheet, which is a pivotal document in the pharmaceutical industry that consolidates essential information about a medication's characteristics, applications, and safety.
Who maintains the CCDS?
The CCDS is centrally maintained by the Marketing Authorization Holder (MAH), ensuring that the company's official position on the product is consistently represented.
What is the purpose of the CCDS?
The purpose of the CCDS is to provide a standardized framework for drug labeling and compliance submissions, which is vital for regulatory adherence and patient safety.
What critical components are included in the CCDS?
The CCDS includes critical components such as indications, contraindications, dosage, pharmacology, and safety information.
How does the CCDS benefit healthcare professionals and regulatory authorities?
The CCDS serves as an invaluable resource for healthcare professionals and regulatory authorities by providing consistent and accurate information about medications.
What challenges are associated with managing the CCDS?
Managing the CCDS presents challenges such as the need to stay updated on policy changes and ensuring compliance with product modifications.
How can AI tools assist in managing the CCDS?
AI tools, such as Trend 483, can streamline the process by identifying patterns in systemic risks and recurrent violations, thereby enhancing the management of updates and compliance efforts.
What are the consequences of non-compliance with CCDS standards?
Non-compliance with established standards can lead to penalties and product recalls, highlighting the importance of adhering to guidelines.
What does the historical evolution of the CCDS indicate?
The historical evolution of the CCDS highlights its essential role in clinical research and reflects a trend towards greater transparency and rigor in pharmaceutical development.




