Overview
Case Report Forms (CRFs) serve as standardized documents that are essential for the collection of participant information in clinical research. Their utilization ensures consistency and precision across studies, addressing a fundamental challenge in the field. Furthermore, CRFs play a critical role in regulatory compliance and data integrity.
The article highlights that electronic CRFs significantly enhance efficiency, reduce error rates, and support the evaluation of new therapies. Consequently, adopting electronic CRFs is not merely a technological upgrade; it is a strategic imperative for advancing clinical research.
Introduction
Understanding the intricacies of clinical research necessitates an in-depth examination of the tools that underpin it, with Case Report Forms (CRFs) emerging as a cornerstone of data collection. These standardized documents not only capture critical participant information but also guarantee consistency and accuracy across diverse research sites.
As the industry transitions towards electronic formats, the stakes are elevated—how can researchers effectively leverage CRFs to bolster data integrity and ensure regulatory compliance amidst evolving standards?
By exploring the multifaceted role of CRFs, we uncover their indispensable significance in the pursuit of reliable and actionable clinical data.
Define Case Report Forms (CRFs) and Their Role in Clinical Research
A Case Report Form (CRF) exemplifies what is CRFs, as it serves as a standardized document crucial for collecting participant information in research studies. It encompasses essential details such as patient demographics, medical history, and treatment outcomes, thereby ensuring consistency and precision across all research sites. With the increasing adoption of electronic case report forms (eCRFs), approximately 70% of research studies now utilize this digital format, significantly enhancing the efficiency and quality of information collection. The pivotal role of case report forms, or what is CRFs, in research cannot be overstated, as they facilitate organized information gathering that is essential for evaluating the safety and effectiveness of new therapies, thus supporting regulatory submissions and scientific assessment.
Regulatory authorities, including the FDA, MHRA, and EMA, stipulate that study information, particularly electronic case report forms, must be securely maintained for 15 to 25 years or longer following the conclusion of a clinical study. This requirement underscores the importance of such forms in ensuring regulatory compliance and long-term data retention. Experts emphasize that well-structured case report forms, often referred to as what is CRFs, are vital for maintaining study integrity and meeting regulatory standards, as they provide a consistent method for documenting adverse events and other critical information.
As Gilbert Hunter notes, case report forms offer a standardized framework for documenting data related to adverse events, aiding researchers and sponsors in evaluating the safety profiles of investigational products. Efficient case report forms not only streamline the information management process but also enhance the overall quality of research studies, ensuring that the information gathered is both reliable and actionable.
Trace the Historical Development of CRFs in Clinical Trials
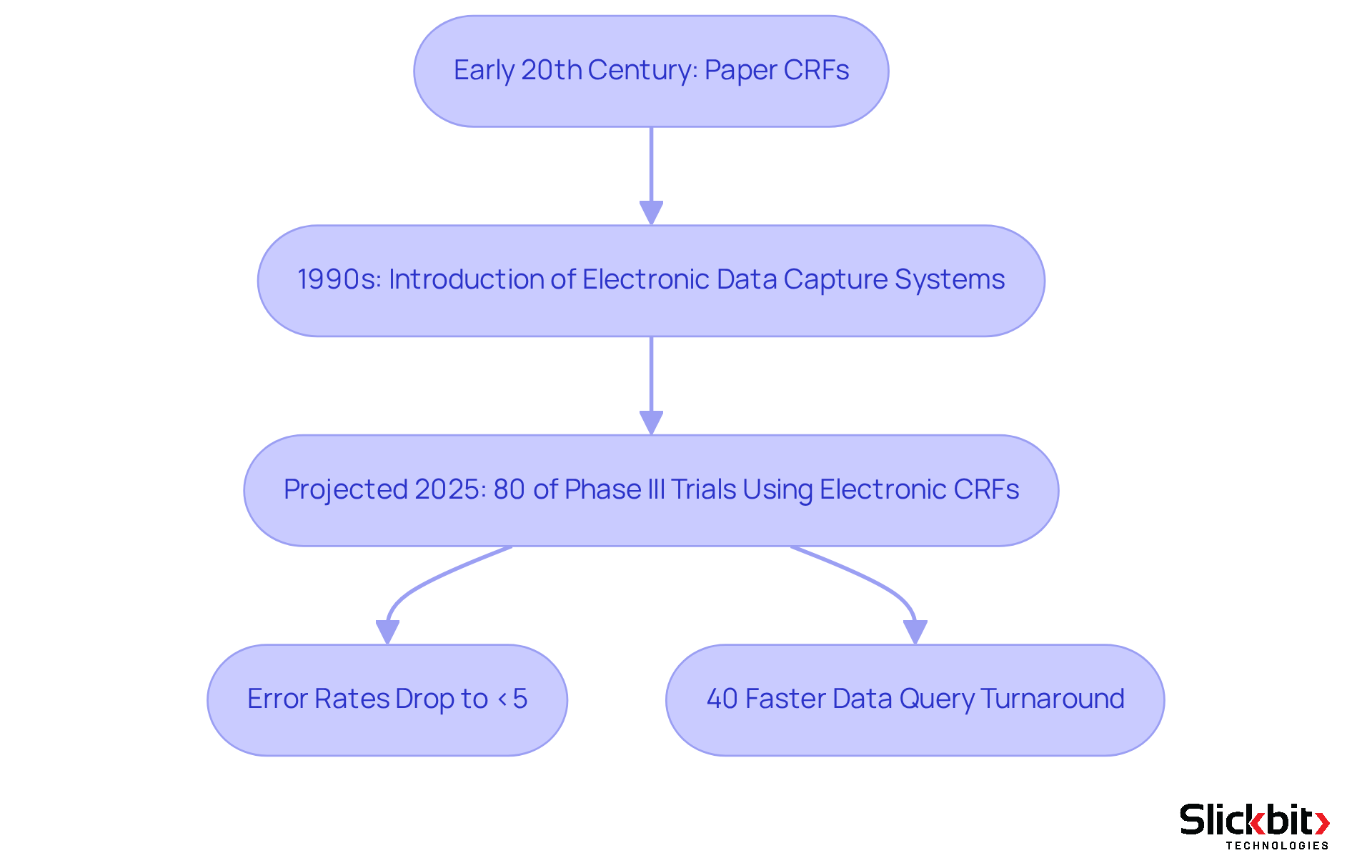
The development of Case Report Forms (CRFs) in research studies began in the early 20th century, which raises the question of what is CRFs and the need for standardized information-gathering techniques. Initially, these forms were rudimentary paper documents designed to capture essential patient information. However, as the complexity of medical research increased and regulatory oversight expanded, the question of what is CRFs experienced significant transformations.
The advent of electronic data capture (EDC) systems in the 1990s revolutionized this process, facilitating efficient data collection, real-time monitoring, and enhanced data integrity. By 2025, it is projected that over 80% of Phase III clinical trials will employ electronic CRFs, underscoring a decisive shift toward digital solutions that bolster compliance with regulatory standards established by agencies such as the FDA and EMA.
This transition not only reduces manual information error rates—lowering them to under 5% for electronic CRFs compared to as high as 26% for traditional paper forms—but also streamlines workflows, ultimately improving patient safety and the quality of information collected. Notably, utilizing electronic CRFs during on-site visits can enhance data query turnaround times by 40%.
The ongoing evolution in research adherence, particularly with the anticipated ICH E6(R3) guidelines, emphasizes the importance of adaptability, ethical considerations, and the integration of advanced technologies in the planning and execution of studies. As the landscape continues to evolve, understanding what is CRFs remains an indispensable element in ensuring the integrity and efficiency of clinical trials, with the potential to foster increased regulatory confidence and enhanced participant safety.
Examine Key Components and Characteristics of Effective CRFs
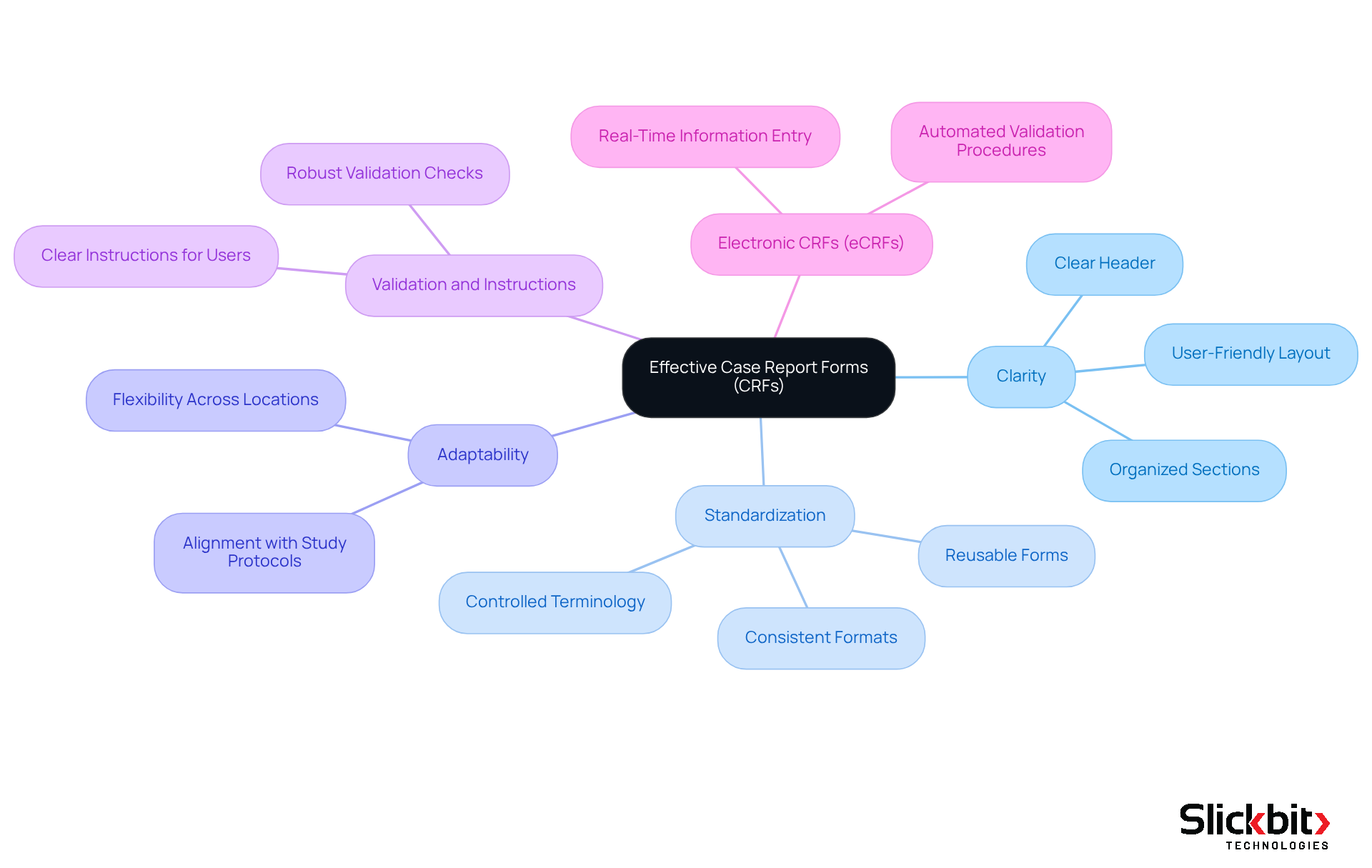
To understand what is CRFs, it is important to know that effective Case Report Forms are defined by several critical components: clarity, standardization, and adaptability. A well-structured CRF, which relates to what is CRFs, must feature:
- A clear header with protocol identifiers
- Organized sections for data entry
- A user-friendly layout that encourages accurate completion
Adaptability is essential, particularly when considering what is CRFs, which must align with various study protocols while ensuring consistency across multiple locations. Key characteristics of what is CRFs include:
- Robust validation checks designed to minimize input errors
- Clear instructions for users
- The capability to capture both qualitative and quantitative information
Furthermore, the implementation of electronic case report forms (eCRFs) has significantly enhanced what is CRFs, facilitating real-time information entry and automated validation procedures. Research indicates that well-constructed case report forms, or CRFs, are essential, and understanding what is CRFs can lead to reduced error rates, with techniques such as double-data entry achieving combined error rates as low as 0.14%, in contrast to elevated rates associated with less organized methods. Consequently, optimal approaches in CRF design clarify what is CRFs by underscoring the significance of clear direction, logical structure, and regulated terminology, which together enhance information quality and adherence to regulatory standards.
Highlight the Importance of CRFs for Regulatory Compliance and Data Integrity
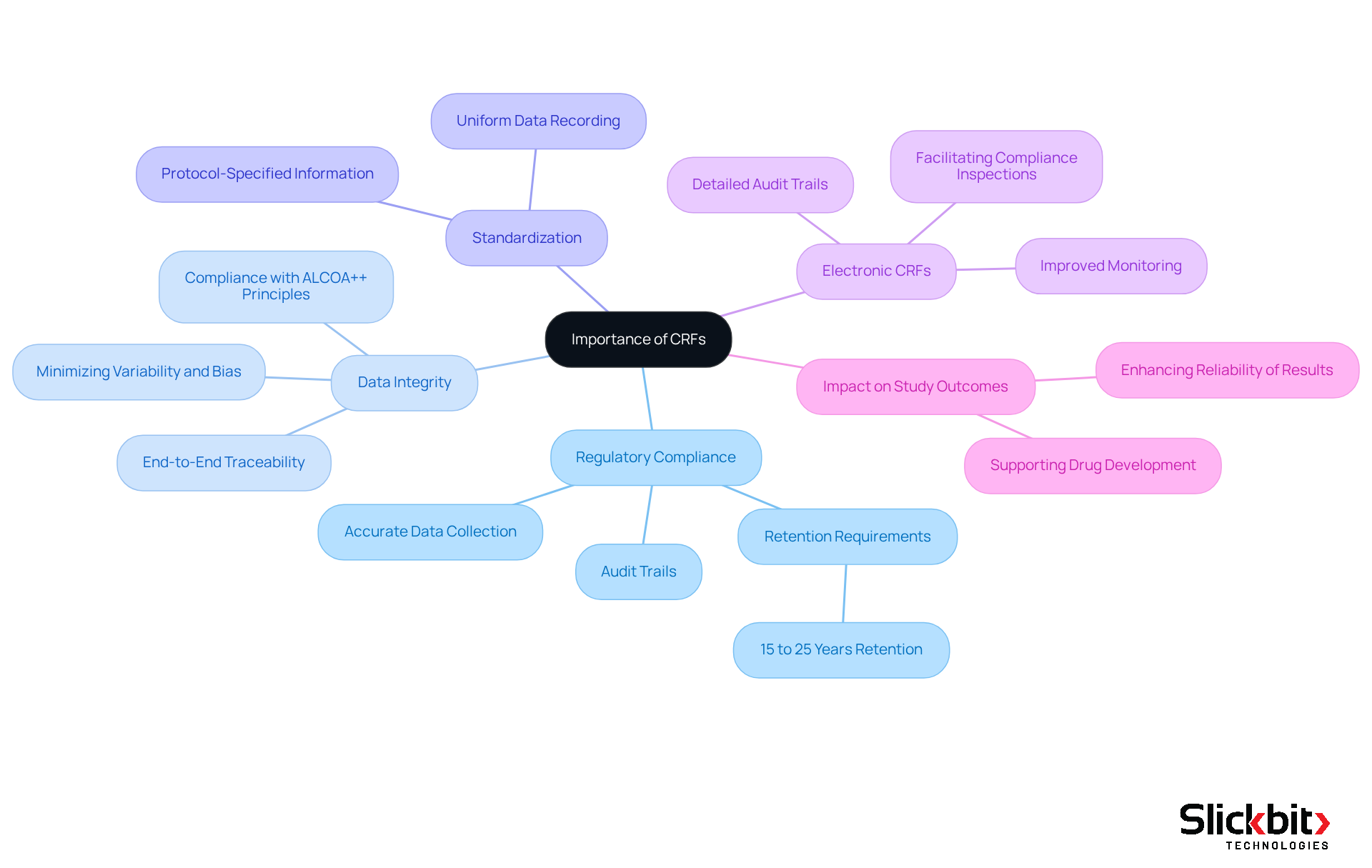
To understand what CRFs are, it is important to note that Case Report Forms are essential for ensuring regulatory compliance and data integrity in research involving human subjects. Regulatory bodies, including the FDA, mandate that all information collected during clinical studies be accurate, comprehensive, and verifiable. Acting as the primary source of information for regulatory submissions, the meticulous design and execution of CRFs are crucial. By standardizing data collection procedures, CRFs minimize variability and bias, significantly enhancing the reliability of study outcomes. Furthermore, the transition to electronic case report forms elevates information management by facilitating improved monitoring of changes and maintaining detailed audit trails, which are vital for demonstrating compliance during regulatory inspections. Ultimately, CRFs play an indispensable role in safeguarding the integrity of clinical trial data and ensuring adherence to rigorous regulatory standards, thereby fostering successful outcomes in drug development.
Conclusion
Understanding Case Report Forms (CRFs) is fundamental to grasping their critical role in clinical research. These standardized documents are essential for collecting and managing participant information, ensuring that data is consistent and reliable across various research sites. Furthermore, the shift towards electronic CRFs has significantly enhanced their efficiency, making them indispensable tools for evaluating the safety and efficacy of new therapies, while also supporting compliance with regulatory standards.
The article highlights several key aspects of CRFs, including their historical evolution from basic paper forms to sophisticated electronic systems. It emphasizes the importance of well-structured CRFs in maintaining data integrity and regulatory compliance, detailing how their design can significantly reduce error rates and streamline the data collection process. In addition, it underscores the necessity of adaptability and clarity in CRF design, which are essential for accurate data entry and effective monitoring of clinical trials.
Ultimately, the significance of CRFs extends beyond mere data collection; they are pivotal in safeguarding the integrity of clinical trial data and ensuring adherence to rigorous regulatory requirements. As the landscape of clinical research continues to evolve, understanding and implementing best practices in CRF design will be crucial for researchers and organizations. Emphasizing the importance of CRFs can lead to improved outcomes in drug development and enhanced participant safety, ultimately benefiting the entire field of clinical research.
Frequently Asked Questions
What is a Case Report Form (CRF)?
A Case Report Form (CRF) is a standardized document used in clinical research to collect participant information, including patient demographics, medical history, and treatment outcomes.
What is the role of CRFs in clinical research?
CRFs play a crucial role in organized information gathering, which is essential for evaluating the safety and effectiveness of new therapies, supporting regulatory submissions, and scientific assessments.
How has the adoption of electronic Case Report Forms (eCRFs) impacted research studies?
Approximately 70% of research studies now utilize eCRFs, significantly enhancing the efficiency and quality of information collection.
What are the regulatory requirements for maintaining CRFs?
Regulatory authorities, such as the FDA, MHRA, and EMA, require that study information, particularly electronic case report forms, be securely maintained for 15 to 25 years or longer after the conclusion of a clinical study.
Why are well-structured CRFs important for clinical studies?
Well-structured CRFs are vital for maintaining study integrity and meeting regulatory standards as they provide a consistent method for documenting adverse events and other critical information.
How do CRFs aid in evaluating the safety profiles of investigational products?
CRFs offer a standardized framework for documenting data related to adverse events, which helps researchers and sponsors assess the safety profiles of investigational products.
What benefits do efficient CRFs provide in research studies?
Efficient CRFs streamline the information management process and enhance the overall quality of research studies, ensuring that the information gathered is reliable and actionable.




