Overview
Quality auditors in life sciences play a pivotal role in ensuring compliance with regulatory standards and maintaining high-quality processes. Their work significantly impacts public health and safety. This article outlines their key responsibilities:
- Conducting audits
- Reporting findings
- Training personnel
Collectively, these duties uphold industry standards and mitigate risks associated with non-compliance. Furthermore, by implementing rigorous auditing procedures, quality auditors not only safeguard public health but also enhance organizational integrity and trust. Consequently, their expertise is indispensable in navigating the complexities of regulatory requirements.
Introduction
In the intricate landscape of life sciences, where precision and compliance are non-negotiable, the role of a quality auditor stands as a cornerstone of operational integrity. These professionals are not only tasked with ensuring adherence to stringent regulatory standards but also with fostering a culture of excellence that safeguards public health. As the demand for skilled quality auditors escalates in response to evolving regulations, a pivotal question arises: what essential skills and responsibilities define their role in maintaining the efficacy and safety of healthcare products? Understanding this dynamic illuminates the critical impact these auditors have on the life sciences sector, ultimately shaping the future of healthcare compliance.
Defining the Role of a Quality Auditor in Life Sciences
A standards assessor in life sciences holds a pivotal role in evaluating and ensuring compliance with regulatory requirements and internal standards across pharmaceuticals, biotechnology, and medical devices. This position is vital in an industry where strict adherence to regulations is paramount for safeguarding public health and safety. Quality auditors conduct systematic reviews and audits, identifying areas of non-compliance, assessing risks, and recommending improvements. Their efforts not only protect public health but also preserve the integrity of the life sciences sector.
Looking ahead to 2025, the demand for skilled evaluators is projected to rise, reflecting the increasing complexity of regulatory standards and the necessity for robust compliance systems. Statistics reveal that organizations with high-performing internal audit functions enjoy enhanced governance and risk awareness; notably, 42% of executives recognize the correlation between effective auditing and improved internal controls. This underscores the significant impact that skilled quality auditors have on regulatory compliance and overall operational excellence within the life sciences sector.
Key Responsibilities of Quality Auditors
Specialists in life sciences play a crucial role in upholding standards and ensuring excellence across various processes. Their key responsibilities encompass several critical areas:
-
Conducting Audits: Regular audits of processes, systems, and documentation are essential for verifying compliance with regulatory requirements and internal standards. Typically, assessment specialists carry out multiple evaluations each month, ensuring ongoing adherence and identifying areas for improvement. They can leverage AI tools like Trend 483 to detect systemic risks and recurring violations, thereby enhancing their auditing processes.
-
Reporting Findings: Following assessments, evaluation specialists compile comprehensive reports that detail their findings, highlighting any discrepancies and providing practical recommendations for corrective actions. Effective audit reports often serve as benchmarks for best practices within the industry, and insights from AI can assist professionals in recognizing trends that require attention.
-
Training Personnel: Quality auditors are pivotal in fostering a culture of compliance by providing training and support to staff regarding performance benchmarks and optimal practices. This training is vital for ensuring that all team members comprehend their roles in maintaining quality. Integrating AI insights into training can further enhance staff understanding of regulatory trends.
-
Monitoring Adherence: Continuous monitoring of compliance with established protocols and regulations is a fundamental responsibility. Quality auditors ensure that any deviations are promptly identified and addressed, thereby mitigating risks associated with non-compliance. AI tools can facilitate real-time monitoring, providing alerts for potential regulatory issues.
-
Collaboration: Effective collaboration with various departments, including R&D, manufacturing, and regulatory affairs, is essential. Standards inspectors strive to embed quality considerations into every operational aspect, ensuring that high standards are a shared responsibility throughout the organization. For example, a Mexican medical device manufacturer faced challenges arising from language barriers when assessing suppliers in China, underscoring the necessity for bilingual personnel in navigating complex compliance environments.
In light of recent developments, such as the National Medical Products Administration's (NMPA) regulatory updates in China, the role of standards assessors has become increasingly significant. The NMPA, as the governing health authority, plays a vital role in ensuring that organizations comply with both local and international standards, which makes the expertise of quality auditors indispensable in this evolving landscape. By utilizing AI tools, professionals can enhance their effectiveness in navigating these complexities.
Essential Skills and Characteristics of Quality Auditors
To excel in their roles, quality auditors in the Life Sciences sector must possess a distinct set of skills and characteristics.
- Attention to detail is paramount; a meticulous eye is crucial for identifying discrepancies and maintaining compliance with regulatory standards.
- Furthermore, analytical skills are essential; the capability to evaluate intricate data and processes is vital for assessing adherence and identifying areas for enhancement. This skill is particularly critical in pharmaceuticals, where data integrity can significantly impact patient safety and product efficacy.
In addition, effective communication skills are necessary for articulating findings and collaborating with various teams within the organization. This ensures that all stakeholders comprehend regulatory needs and excellence benchmarks.
- Problem-solving skills are also indispensable; inspectors must be adept at recognizing issues and proposing practical solutions to enhance standards and adherence. For instance, during audits, they may uncover inefficiencies in manufacturing processes and recommend adjustments that lead to improved operational performance.
Moreover, a thorough understanding of industry regulations, such as Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP), is essential for maintaining adherence. This knowledge enables examiners to efficiently assess compliance with essential standards, thereby enhancing assurance procedures.
Collectively, these skills not only support the integrity of the Life Sciences sector but also contribute to the overarching goal of ensuring safety and efficacy in pharmaceutical products.
The Importance of Quality Auditors in Life Sciences Organizations
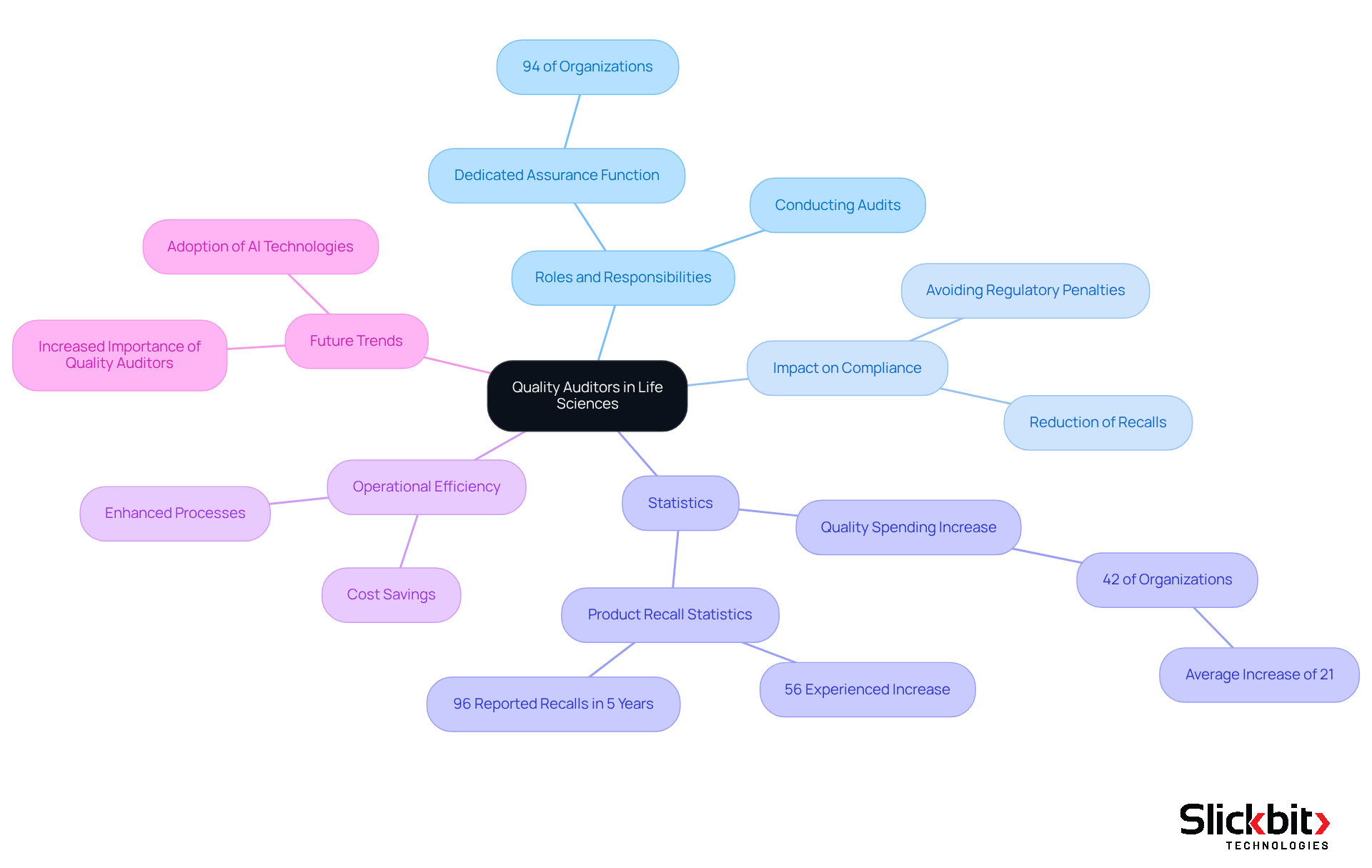
Quality auditors play a pivotal role in life sciences organizations, ensuring that products and processes meet the highest standards of excellence and compliance. Their diligent efforts significantly reduce the risk of costly recalls, regulatory penalties, and reputational damage linked to non-compliance. Notably, 94% of survey participants report having a dedicated assurance function in place, emphasizing the critical nature of this role.
By conducting thorough audits, quality inspectors not only safeguard public health but also foster a culture of excellence that drives ongoing improvement initiatives. As Bermingham, Castleman & Pierce Inc. aptly stated, "Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution." This proactive approach enhances operational efficiency, ultimately yielding substantial cost savings in pharmaceuticals.
Furthermore, with the advent of AI technologies like Trend 483, assessment professionals can leverage advanced tools to identify patterns in systemic risks and recurring violations from FDA inspections. This capability simplifies regulatory processes and aids reviewers in making informed decisions that enhance operational efficiency.
With 42% of organizations increasing quality spending at an average rate of 21%, the role of quality auditors is expected to become even more crucial in 2025, positioning them as key players in maintaining the integrity and efficacy of healthcare products amid escalating compliance demands.
Conclusion
Quality auditors in the life sciences sector are essential for ensuring that products and processes meet stringent standards of compliance and safety. Their role transcends basic evaluation; they actively shape the integrity of the industry, safeguarding public health while driving operational excellence. As regulatory frameworks become increasingly complex, the demand for skilled quality auditors is expected to rise, underscoring the critical nature of their contributions.
This exploration has highlighted the key responsibilities of quality auditors, which include:
- Conducting thorough audits
- Reporting findings
- Training personnel
- Monitoring adherence
- Fostering collaboration across departments
Each of these functions is vital for maintaining compliance and enhancing the overall quality of life sciences operations. The integration of advanced technologies, such as AI, empowers auditors to identify risks and streamline processes, further reinforcing their value in the industry.
Given these insights, it is evident that the role of quality auditors will grow in importance as organizations strive to meet escalating compliance demands. Investing in the development of skilled auditors not only mitigates risks associated with non-compliance but also cultivates a culture of excellence that benefits the entire healthcare landscape. The future of life sciences depends on these dedicated professionals who ensure that quality is not merely an aspiration but a consistent reality.
Frequently Asked Questions
What is the role of a quality auditor in life sciences?
A quality auditor in life sciences evaluates and ensures compliance with regulatory requirements and internal standards across pharmaceuticals, biotechnology, and medical devices.
Why is the role of a quality auditor important?
This role is vital for safeguarding public health and safety by ensuring strict adherence to regulations and conducting systematic reviews and audits to identify non-compliance and recommend improvements.
What are the main responsibilities of a quality auditor?
Quality auditors conduct systematic reviews and audits, identify areas of non-compliance, assess risks, and recommend improvements to enhance compliance and operational excellence.
What is the projected demand for quality auditors by 2025?
The demand for skilled evaluators is projected to rise by 2025 due to the increasing complexity of regulatory standards and the necessity for robust compliance systems.
How do high-performing internal audit functions impact organizations?
Organizations with high-performing internal audit functions enjoy enhanced governance and risk awareness, with 42% of executives recognizing a correlation between effective auditing and improved internal controls.
What is the significance of skilled quality auditors in the life sciences sector?
Skilled quality auditors significantly impact regulatory compliance and overall operational excellence within the life sciences sector, helping to protect public health and preserve the integrity of the industry.




