Overview
The Investigational Medicinal Product Dossier (IMPD) serves as a pivotal compliance document, delivering comprehensive information about investigational medicinal products intended for clinical trials. It is systematically divided into:
- Quality
- Safety and Efficacy
A well-prepared IMPD significantly elevates the chances of successful Clinical Trial Applications by adhering to regulatory standards and fostering effective communication among stakeholders. Consequently, this meticulous preparation ultimately facilitates the timely initiation of clinical trials.
Introduction
The Investigational Medicinal Product Dossier (IMPD) is a cornerstone for the success of clinical trials, encapsulating crucial information about investigational products. Its meticulous structure not only ensures compliance with regulatory standards but also significantly enhances the likelihood of timely trial initiation.
However, the evolving pharmaceutical landscape presents a pressing challenge: how can researchers adeptly navigate the complexities of the IMPD to circumvent common pitfalls that often lead to delays? Grasping the intricacies of this essential document is vital for those committed to advancing innovative therapies while prioritizing patient safety.
Define the Investigational Medicinal Product Dossier (IMPD)
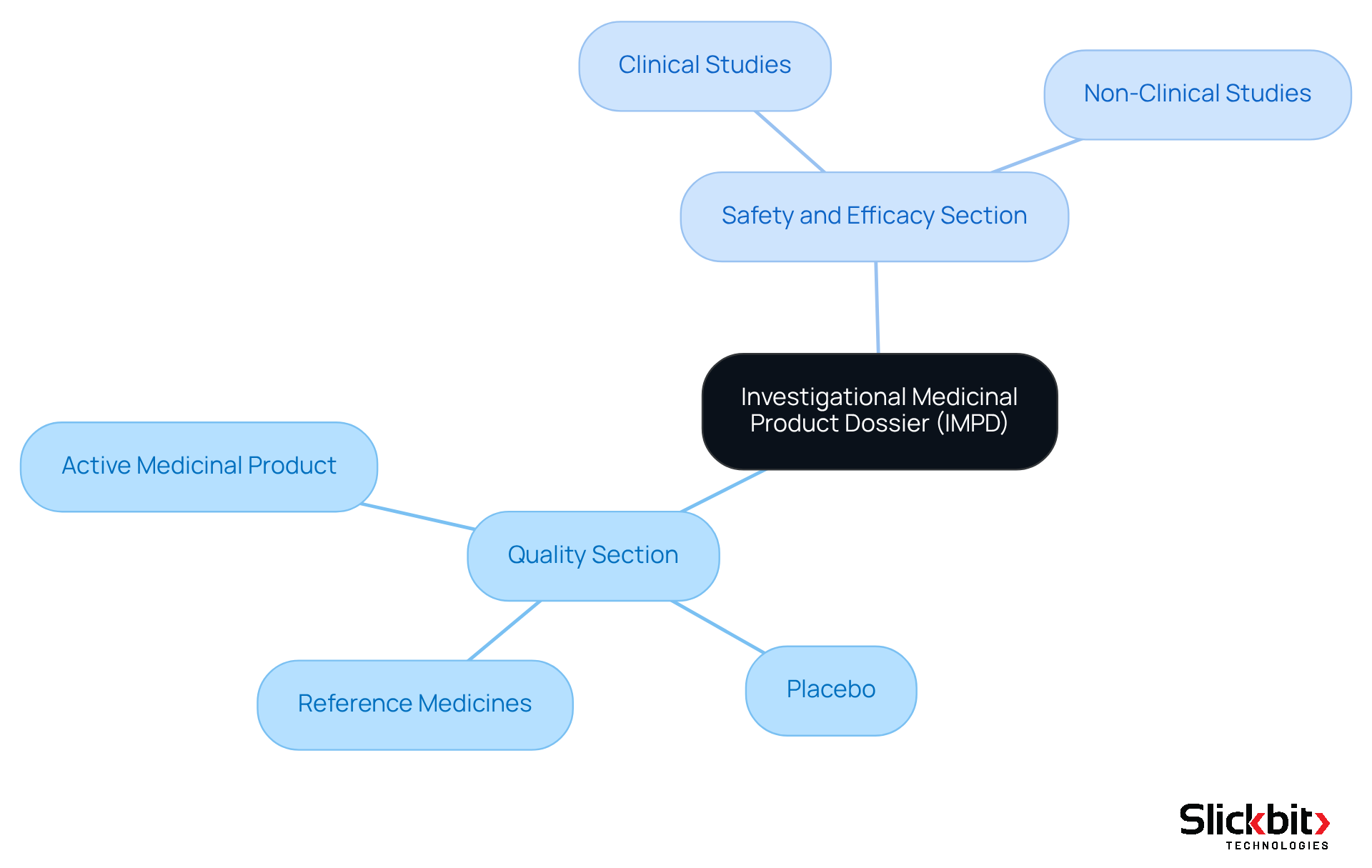
The investigational medicinal product dossier is a pivotal compliance document that provides comprehensive information about an investigational medicinal product intended for clinical trials. It is systematically divided into two primary sections:
- The Quality section
- The Safety and Efficacy section
The Quality section delineates the active medicinal product, any placebo utilized, and relevant reference medicines, ensuring adherence to stringent manufacturing and control standards. This compliance is essential, as it guarantees that the product meets the necessary safety and efficacy benchmarks. Conversely, the Safety and Efficacy section encapsulates data from all clinical and non-clinical studies, delivering an exhaustive evaluation of the associated risks and benefits. This dual structure is vital for oversight bodies, enabling them to assess the product's suitability for human trials and establishing the investigational medicinal product dossier as a cornerstone of the Clinical Trial Application (CTA) process.
As we approach 2025, it is imperative for pharmaceutical R&D managers to stay informed about specific updates to investigational medicinal product regulatory requirements. These changes are crucial for maintaining compliance and facilitating successful trial initiation. This underscores the importance of meticulous documentation and data presentation in the regulatory submission process.
Contextualize the IMPD in Clinical Trials
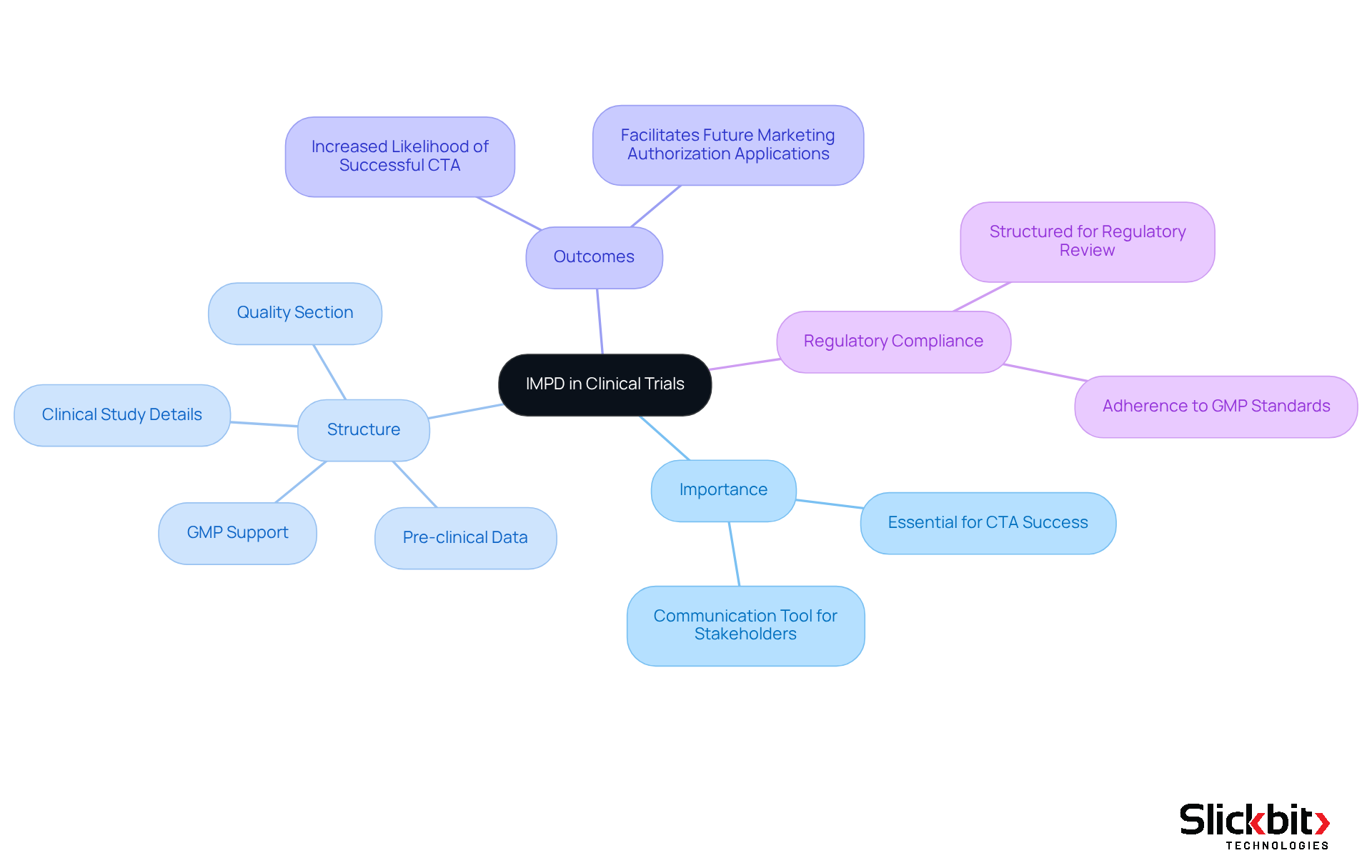
The Investigational Medicinal Product Document is essential for the clinical trial process, particularly within the European Union, where it is a prerequisite for obtaining permission to conduct trials involving Investigational Medicinal Products (IMPs). This compliance document provides a structured overview of the investigational product, outlining its intended use, manufacturing processes, and quality control measures. A meticulously prepared investigational medicinal product dossier significantly increases the chances of successful Clinical Trial Applications (CTAs), as over 70% of research studies encounter delays due to documentation-related issues, underscoring the critical need for comprehensive documentation.
Furthermore, the document serves as a vital communication tool between sponsors and oversight bodies, ensuring that all ethical considerations are addressed and participant safety is prioritized. As Annegret Wiedemann noted, "The value added from the clear, precise, and creatively proactive writing of the document is essential throughout all phases of development." By organizing essential information in an accessible format, the organization fosters transparency and collaboration among stakeholders, including researchers, governing bodies, and patients. This alignment is crucial for the effective execution of clinical trials and the progression of innovative therapies.
The document is systematically divided into four sections summarizing quality, pre-clinical, and clinical study details, which are indispensable for compliance review. Additionally, the Indianapolis Metropolitan Police Department must be supported by Good Manufacturing Practice (GMP) documentation to ensure regulatory adherence. Recent data indicates that a well-structured investigational medicinal product dossier not only streamlines the approval process but also facilitates future marketing authorization applications (MAAs), highlighting its importance in the drug development landscape.
Outline Key Components of the IMPD
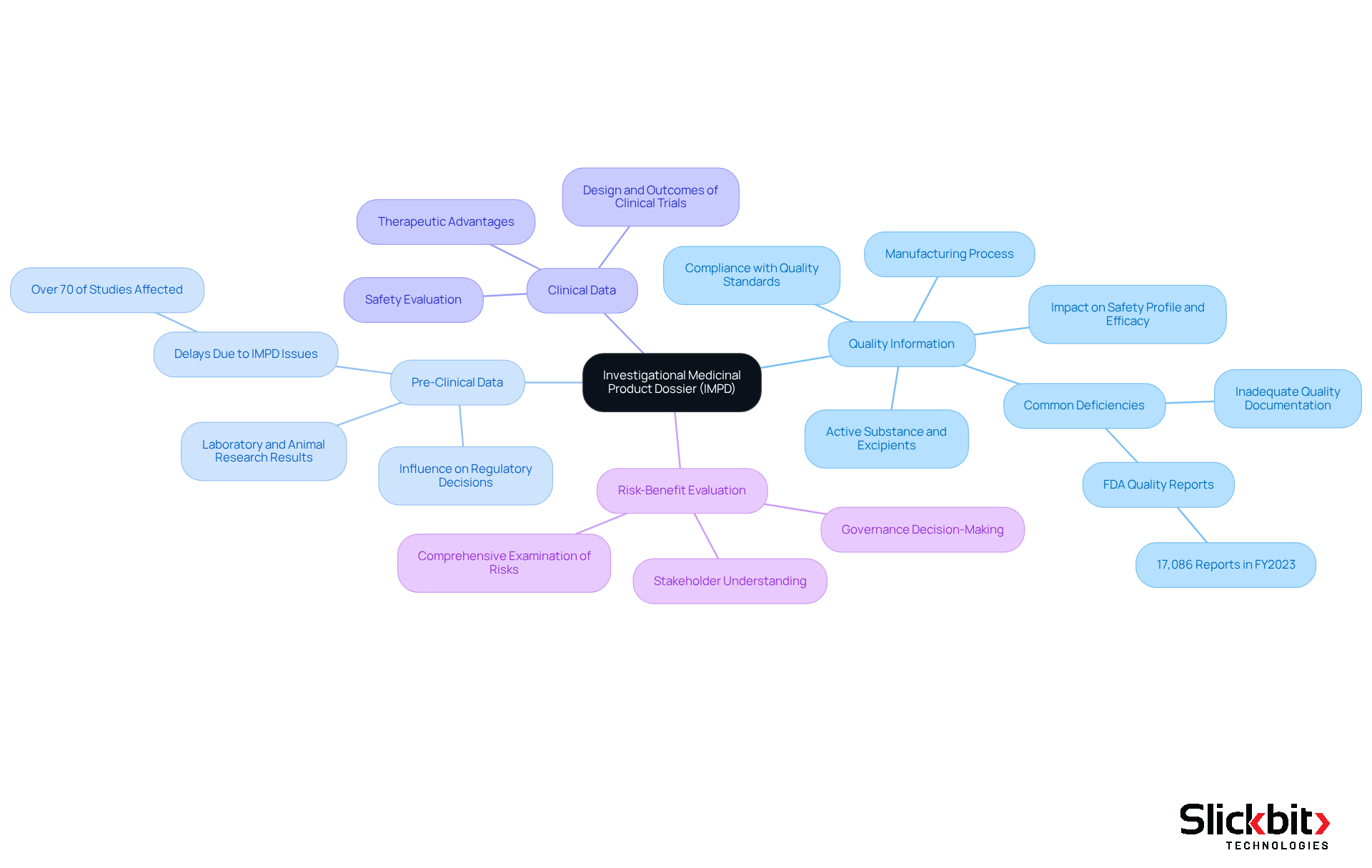
The Investigational Medicinal Product Dossier (IMPD) is structured into four essential sections, each playing a pivotal role in the regulatory approval process:
-
Quality Information: This section details the active substance, excipients, and the manufacturing process, ensuring compliance with stringent quality standards. A well-structured Quality Information section is essential for showcasing the item's safety profile and efficacy, which are important in clinical research. Recent statistics indicate that common deficiencies in the investigational medicinal product dossier submissions often stem from inadequate quality documentation, underscoring the need for thorough preparation. In fact, the FDA received 17,086 quality-related reports in FY2023, highlighting ongoing quality issues in the pharmaceutical industry. As pointed out by the Bioaccess Content Team, "A carefully assembled investigational medicinal item dossier significantly enhances the chances of favorable research outcomes."
-
Pre-Clinical Data: This section showcases results from laboratory and animal research that assess the safety and effectiveness of the investigational medicinal item (IMP). Successful trials often showcase robust pre-clinical data, which can significantly influence regulatory decisions and expedite the approval process. Significantly, over 70% of research studies face delays due to issues related to their investigational medicinal product dossier, highlighting the necessity of careful planning and documentation.
-
Clinical Data: This section outlines the design and outcomes of clinical trials conducted to evaluate the performance of the item in human subjects. Thorough clinical information is crucial for showcasing the therapeutic advantages and safety of the IMP, in accordance with compliance expectations.
-
Risk-Benefit Evaluation: The final element offers a comprehensive examination of the overall risk compared to the advantage of the investigational item. This evaluation is essential for governance decision-making, as it assists stakeholders in comprehending the potential effects of the product in practical uses.
Each part of the investigational medicinal product dossier must be meticulously prepared to meet regulatory expectations, as a well-structured investigational medicinal product dossier can lead to quicker approvals by anticipating and addressing potential compliance issues. Insights from pharmaceutical experts emphasize that quality information is not merely a formality but a cornerstone of successful drug development, enhancing the likelihood of favorable study outcomes. As Peter Drucker aptly stated, "There is nothing so useless as doing efficiently that which should not be done at all," highlighting the importance of quality over mere efficiency in the project.
Highlight the Importance of the IMPD in Clinical Research
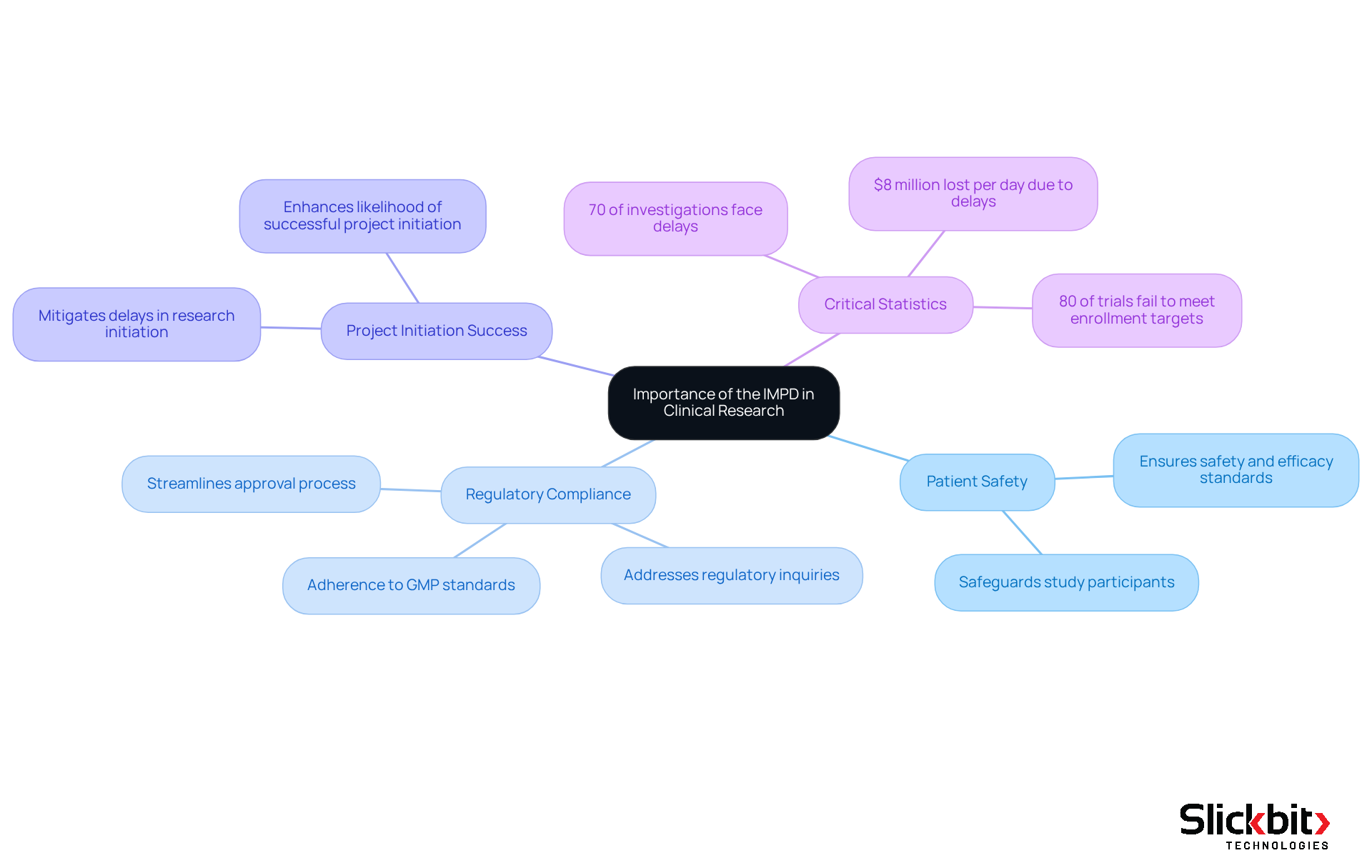
The Investigational Medicinal Product Document (IMPD) is essential for the advancement of investigational products, prioritizing patient safety and compliance with regulations. A well-structured clinical trial application not only streamlines oversight by regulatory bodies but significantly enhances the likelihood of successful project initiation.
Research indicates that over 70% of clinical investigations face delays due to issues related to their investigational medicinal product dossier, while approximately 80% of clinical trials fail to meet initial enrollment targets and timelines, highlighting the critical role of the investigational medicinal product dossier in expediting the approval process.
Furthermore, the document serves as a central reference point for all stakeholders involved in a research project, ensuring alignment on the objectives and methodologies of clinical trials. The quality of the submission is directly linked to approval timelines; a meticulously prepared submission mitigates delays in research initiation by proactively addressing regulatory inquiries.
This positions the IMPD as a cornerstone in the clinical research landscape, playing a pivotal role in the overall success of drug development programs. As Katherine Ruiz, an expert in compliance issues, emphasizes, the thoroughness of the department is crucial for safeguarding study participants and ensuring that investigational products meet safety and efficacy standards.
The IMPD comprises sections detailing the active ingredient, placebo, reference medications, quality, manufacturing processes, and control measures, all of which are vital for regulatory compliance.
Conclusion
The Investigational Medicinal Product Dossier (IMPD) stands as a pivotal document in the domain of clinical trials, encapsulating essential information regarding an investigational medicinal product. Its structured format, systematically divided into quality, safety, and efficacy sections, guarantees that all critical aspects are thoroughly addressed. This organization allows regulatory bodies to effectively evaluate the product's suitability for human trials. Beyond mere compliance, the IMPD plays an instrumental role in facilitating successful Clinical Trial Applications (CTAs) and advancing innovative therapies.
Key insights throughout the article underscore the necessity of a meticulously prepared IMPD. Each section, from detailed quality information to comprehensive pre-clinical and clinical data, is vital in the regulatory approval process. Notably, statistics reveal that over 70% of clinical investigations encounter delays due to documentation issues, reinforcing the need for thorough preparation and clear communication among stakeholders involved in clinical research.
Given these considerations, it becomes evident that the IMPD transcends a mere regulatory requirement; it is a cornerstone of successful drug development. As the pharmaceutical landscape continues to evolve, R&D managers must stay abreast of regulatory changes and ensure meticulous documentation. By emphasizing the significance of the IMPD, the potential for more efficient clinical trials increases, ultimately enhancing patient safety and facilitating the successful introduction of new therapies into the market.
Frequently Asked Questions
What is the Investigational Medicinal Product Dossier (IMPD)?
The Investigational Medicinal Product Dossier (IMPD) is a compliance document that provides comprehensive information about an investigational medicinal product intended for clinical trials.
What are the main sections of the IMPD?
The IMPD is systematically divided into two primary sections: the Quality section and the Safety and Efficacy section.
What information is included in the Quality section of the IMPD?
The Quality section delineates the active medicinal product, any placebo utilized, and relevant reference medicines, ensuring adherence to stringent manufacturing and control standards.
Why is compliance in the Quality section important?
Compliance in the Quality section is essential as it guarantees that the product meets the necessary safety and efficacy benchmarks.
What does the Safety and Efficacy section of the IMPD encompass?
The Safety and Efficacy section encapsulates data from all clinical and non-clinical studies, providing an exhaustive evaluation of the associated risks and benefits.
Why is the dual structure of the IMPD important?
The dual structure is vital for oversight bodies as it enables them to assess the product's suitability for human trials and establishes the IMPD as a cornerstone of the Clinical Trial Application (CTA) process.
What should pharmaceutical R&D managers be aware of as we approach 2025?
Pharmaceutical R&D managers should stay informed about specific updates to investigational medicinal product regulatory requirements, which are crucial for maintaining compliance and facilitating successful trial initiation.
What is the significance of meticulous documentation in the regulatory submission process?
Meticulous documentation and data presentation are crucial in the regulatory submission process to ensure compliance and support the successful initiation of clinical trials.




