Overview
The role of a clinical data specialist is pivotal within the pharmaceutical industry, primarily concerning the management and validation of research data. This function is essential for ensuring accuracy and compliance with regulatory standards. Key skills such as:
- Analytical abilities
- Attention to detail
- Proficiency in data management tools
are vital for supporting drug development and enhancing the efficiency of regulatory processes. Consequently, these specialists play a crucial role in safeguarding public health, demonstrating their importance in the industry's landscape.
Introduction
In the intricate realm of pharmaceutical research, the role of a clinical data specialist stands as a cornerstone of successful drug development. These professionals are entrusted with the critical responsibility of ensuring the accuracy and integrity of data, which directly impacts the safety and efficacy of new medications. As clinical trials become increasingly complex, the demand for skilled specialists who can adeptly navigate regulatory landscapes and implement effective data management strategies continues to rise.
What challenges do these experts encounter in their pursuit of maintaining data quality? Furthermore, how do their skills evolve to meet the ever-changing demands of the industry?
Define Clinical Data Specialist: Key Functions and Responsibilities
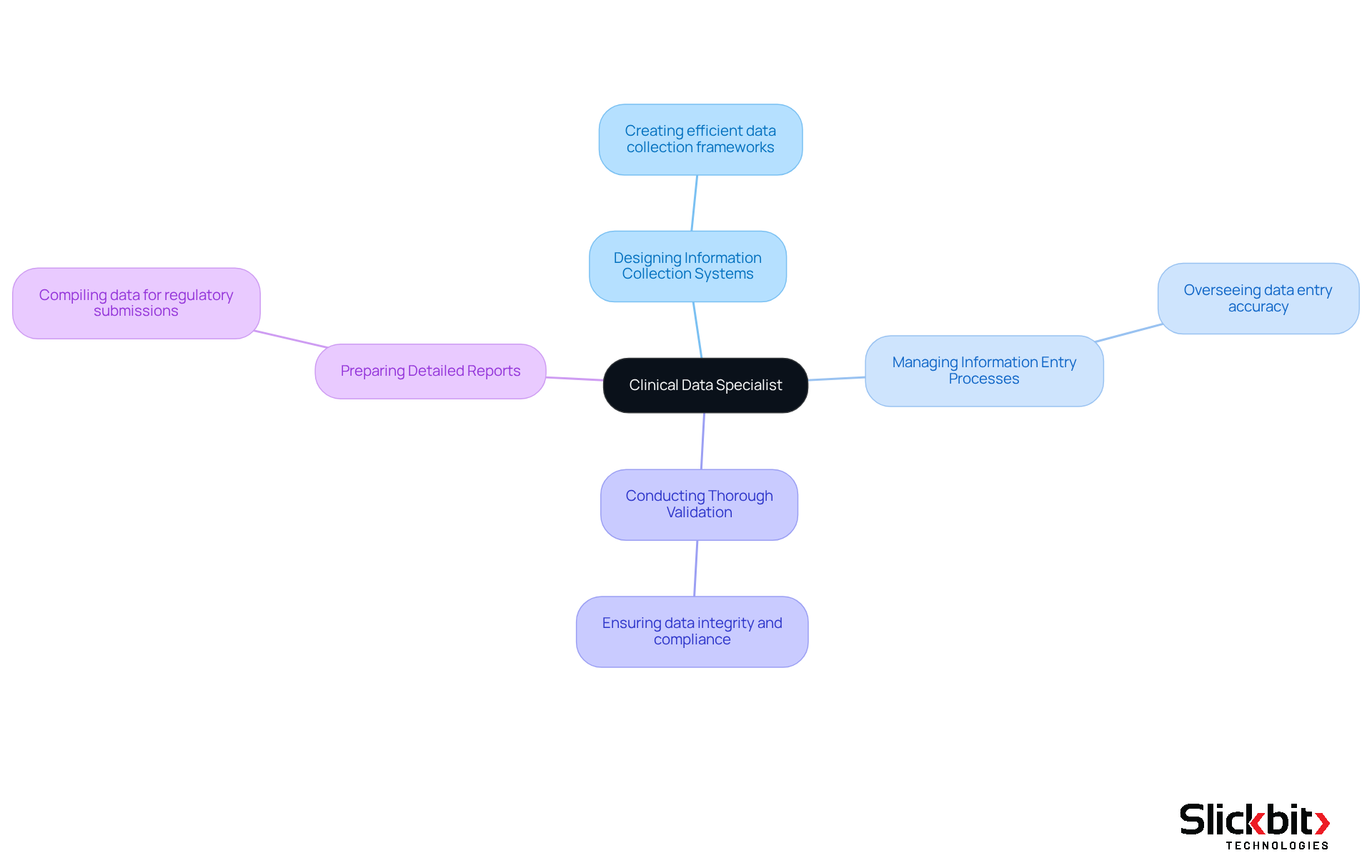
A clinical data specialist plays a pivotal role in the pharmaceutical industry, overseeing and evaluating research information to guarantee its precision, integrity, and compliance with regulatory standards. Their primary responsibilities encompass:
- Designing information collection systems
- Managing information entry processes
- Conducting thorough validation
- Preparing detailed reports for regulatory submissions
Furthermore, the role of a clinical data specialist is instrumental in ensuring that the data gathered during research studies is reliable, supporting the safety and efficacy claims of new medications. This position is essential for a clinical data specialist to maintain the quality of information that informs decision-making in drug development, ultimately safeguarding public health and advancing medical innovation.
Contextualize the Role: Importance in Pharmaceutical Research and Development
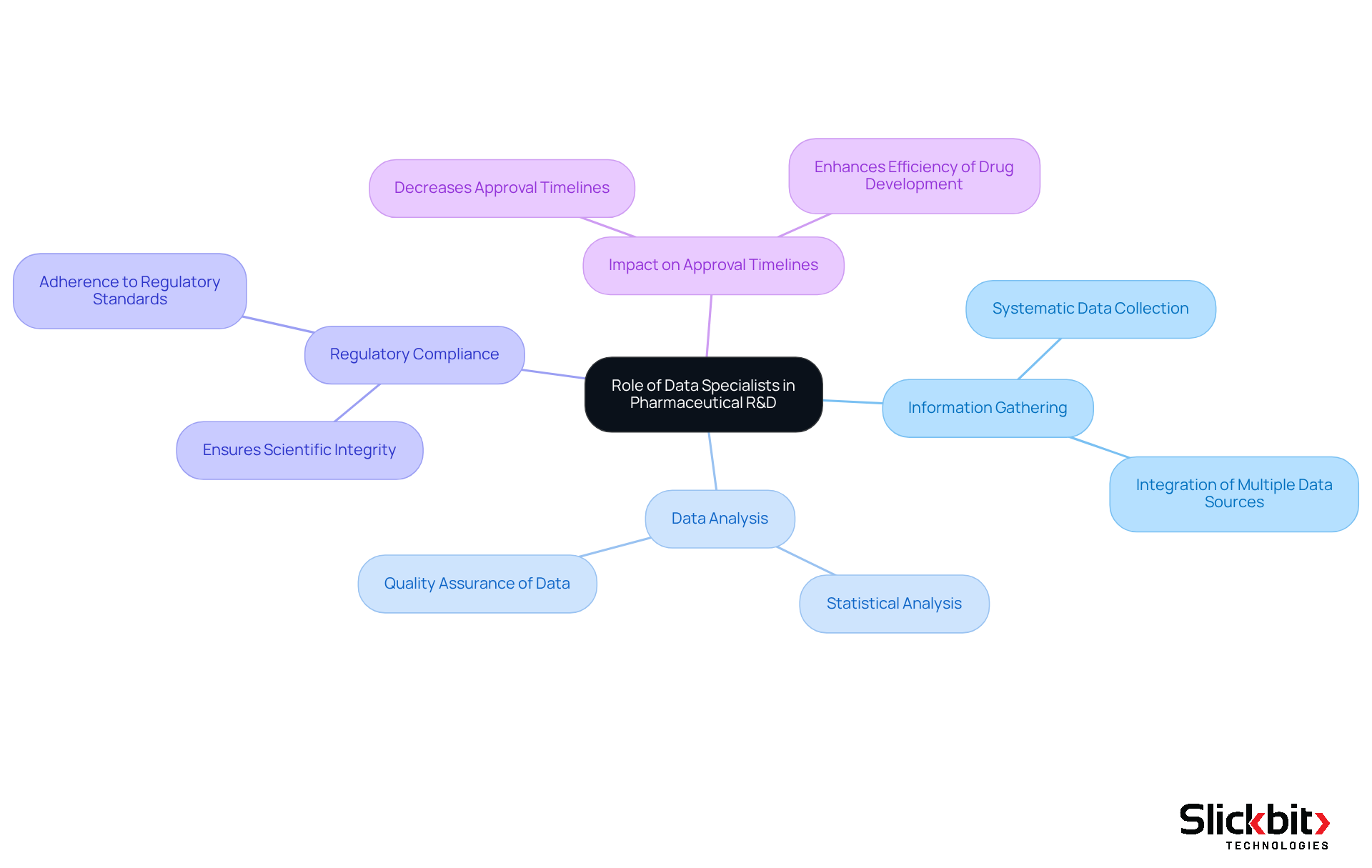
In the dynamic landscape of pharmaceutical research and development, Data Specialists play a pivotal role in the success of trials. Their expertise is vital for systematic information gathering and precise analysis, both of which are essential for regulatory approval. As drug development becomes increasingly intricate, the importance of Data Specialists has escalated, functioning as an essential connection between healthcare operations and information management. This ensures that the information generated not only complies with regulatory standards but also maintains the scientific integrity of the studies. This dual focus on compliance and scientific validity directly impacts the speed and efficiency of introducing new therapies to the market, underscoring their indispensable presence in the pharmaceutical sector.
Research has demonstrated that efficient information management practices can significantly decrease approval timelines, highlighting how clinical data specialists can impact regulatory processes. Their role is additionally supported by professional views emphasizing the importance of high-quality information in attaining successful results in medical studies. Significantly, the average total duration to carry out research studies and finalize the approval phase rose by 4.8 months from 2008-13 to 2014-18, emphasizing the significance of efficient data management in reducing such delays. Furthermore, perspectives from industry specialists, including Jim Kremidas, emphasize the necessity for skilled professionals like Data Specialists to improve the efficiency and quality of the research workforce, which is vital in reversing the trend of extended research timelines.
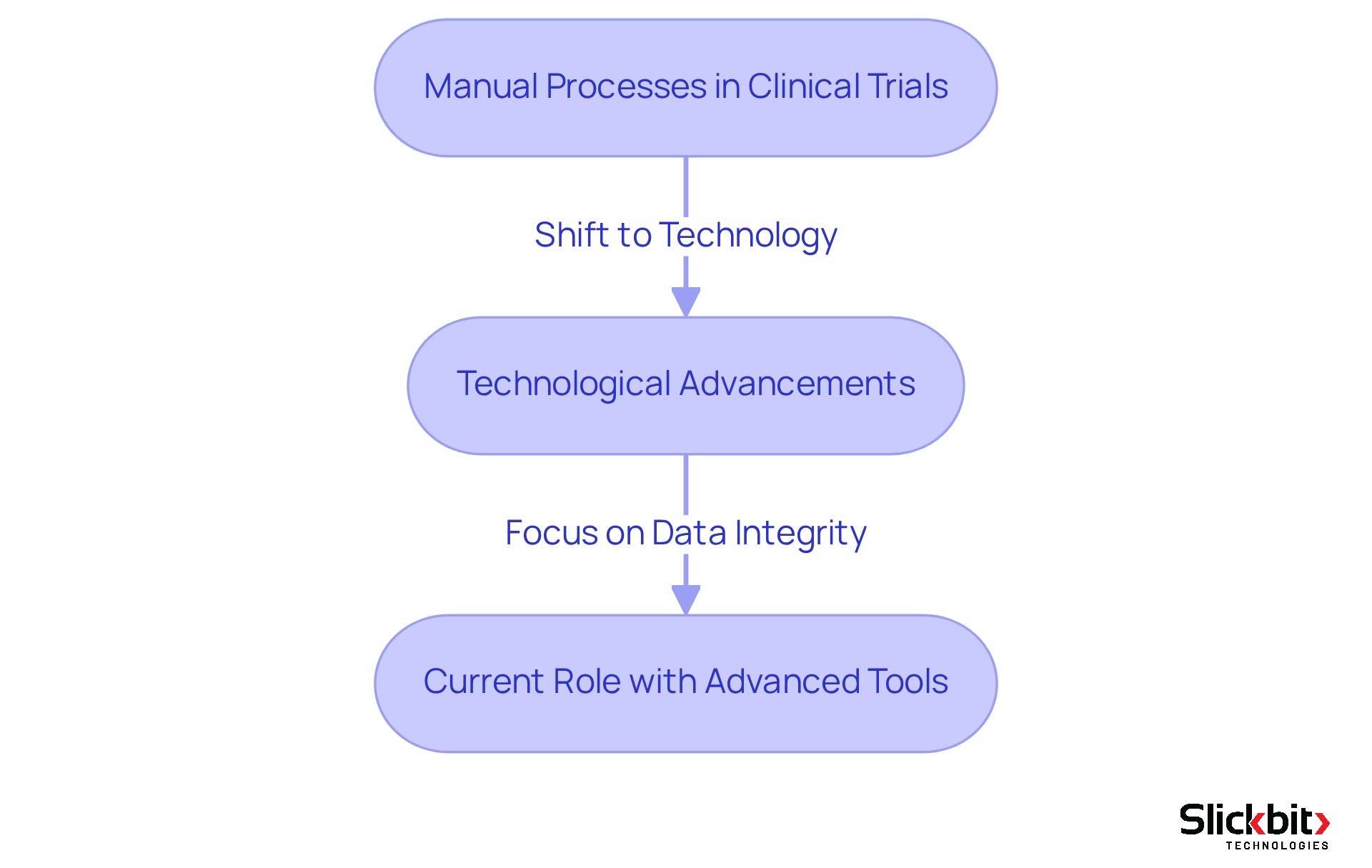
Trace the Evolution: Historical Development of Clinical Data Specialists
The role of a clinical data specialist has undergone a remarkable transformation over the past few decades. Initially, information management in clinical trials relied heavily on manual processes, which were often plagued by errors and inefficiencies. However, with the advent of technology and electronic information capture systems in the late 1990s and early 2000s, this role began to shift towards a more analytical and technology-driven focus. Furthermore, the introduction of regulatory frameworks, such as Good Clinical Practice (GCP), underscored the necessity for specialized roles dedicated to ensuring data integrity and compliance. Today, clinical data specialists are equipped with advanced tools and methodologies that empower them to effectively manage extensive datasets and ensure adherence to stringent regulatory requirements in clinical trials.
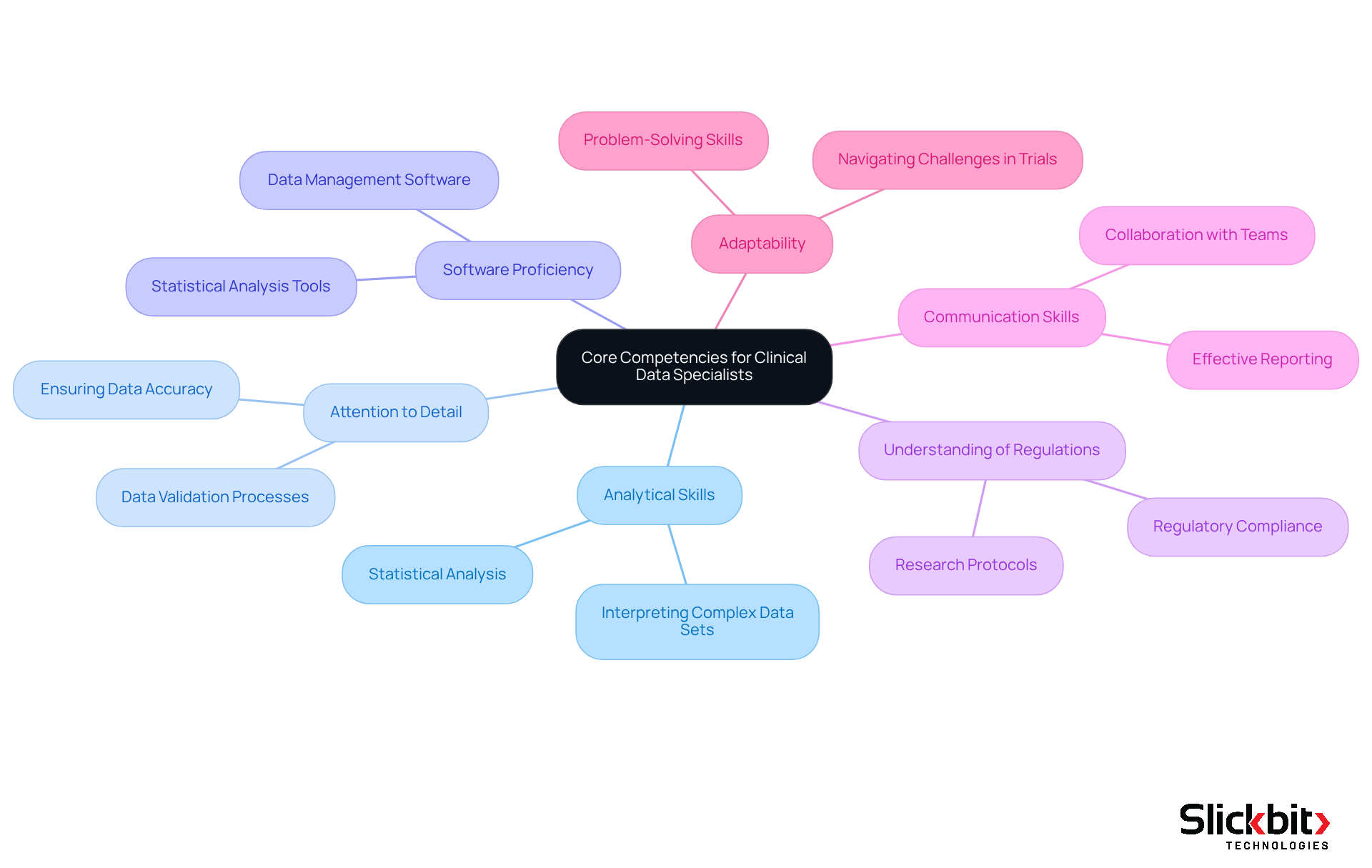
Identify Core Competencies: Skills Essential for Success
Several core competencies are essential to excel as a clinical data specialist. Strong analytical skills are crucial for a clinical data specialist when interpreting complex data sets, while attention to detail ensures data accuracy. Proficiency in data management software and statistical analysis tools is also necessary for a clinical data specialist. Additionally, a comprehensive understanding of regulatory obligations and research protocols is vital for a clinical data specialist. Communication abilities play a significant role, as clinical data specialists frequently collaborate with cross-functional teams, including operational tasks, biostatistics, and regulatory affairs. Furthermore, a clinical data specialist must demonstrate adaptability and problem-solving skills to navigate the challenges that arise during clinical trials, ensuring that data integrity is maintained throughout the process.
Conclusion
The role of a clinical data specialist is integral to the pharmaceutical industry, serving as a critical linchpin in the management and analysis of research data. This position not only ensures the accuracy and integrity of clinical information but also upholds compliance with stringent regulatory standards. By effectively overseeing data collection systems and validating information, clinical data specialists play a vital role in supporting the development of safe and effective medications.
Key points throughout the article have highlighted the importance of clinical data specialists in streamlining pharmaceutical research and development processes. Their expertise in data management significantly impacts the efficiency of clinical trials, reducing approval timelines and enhancing the quality of research outcomes. The evolution of this role, from manual processes to a technology-driven approach, underscores the necessity for skilled professionals adept in both analytical and regulatory aspects of clinical data management.
In a landscape where the demand for innovative therapies continues to grow, the significance of clinical data specialists cannot be overstated. Their contributions not only facilitate regulatory approvals but also advance public health initiatives. As the pharmaceutical field becomes increasingly complex, fostering a deeper understanding of the essential skills and competencies required for these professionals is crucial. Embracing the role of clinical data specialists is a step toward ensuring the integrity of medical research and the safety of new treatments.
Frequently Asked Questions
What is the role of a clinical data specialist?
A clinical data specialist oversees and evaluates research information in the pharmaceutical industry to ensure its precision, integrity, and compliance with regulatory standards.
What are the key responsibilities of a clinical data specialist?
Key responsibilities include designing information collection systems, managing information entry processes, conducting thorough validation, and preparing detailed reports for regulatory submissions.
Why is the role of a clinical data specialist important in drug development?
The role is important because it ensures that the data gathered during research studies is reliable, supporting the safety and efficacy claims of new medications and ultimately safeguarding public health.
How does a clinical data specialist contribute to medical innovation?
By maintaining the quality of information that informs decision-making in drug development, clinical data specialists help advance medical innovation.




