Overview
This article underscores the critical importance, evolution, and essential components of Medical Device Reporting (MDR) within the realm of medical products and patient safety. MDR serves as a vital mechanism for manufacturers to report adverse events and ensure compliance, thereby significantly enhancing patient safety through systematic monitoring and accountability. Furthermore, the integration of AI technologies is revolutionizing reporting and risk management, paving the way for improved outcomes. This evolution not only reflects the changing landscape of healthcare but also emphasizes the necessity for manufacturers to adapt and innovate in their reporting practices.
Introduction
MDR medical reporting stands at the forefront of patient safety within the pharmaceutical industry, serving as a critical regulatory mechanism for manufacturers and importers of medical products. By ensuring the timely reporting of adverse events and product issues, MDR not only safeguards public health but also enhances the accountability of manufacturers.
As the landscape of medical device regulations evolves, companies face the pressing challenge of navigating the complexities of compliance. Furthermore, they must leverage new technologies to improve their reporting processes effectively. This dual focus on regulatory adherence and technological advancement is essential for fostering a safer healthcare environment.
Define MDR Medical Reporting
MDR médical reporting, which is also referred to as medical product reporting, represents a critical regulatory obligation for manufacturers and importers of medical products to report adverse events and product issues to regulatory authorities. This process is vital for ensuring patient safety and maintaining the integrity of medical instruments compliant with MDR médical available in the market. MDR encompasses a range of activities, including the collection, analysis, and communication of information pertaining to equipment performance and safety. Such measures facilitate timely interventions when issues arise. Furthermore, the integration of AI technology can significantly enhance this process by identifying trends in compliance and systemic risks, ultimately improving the efficiency and effectiveness of MDR documentation.
Contextualize the Importance of MDR in Pharma
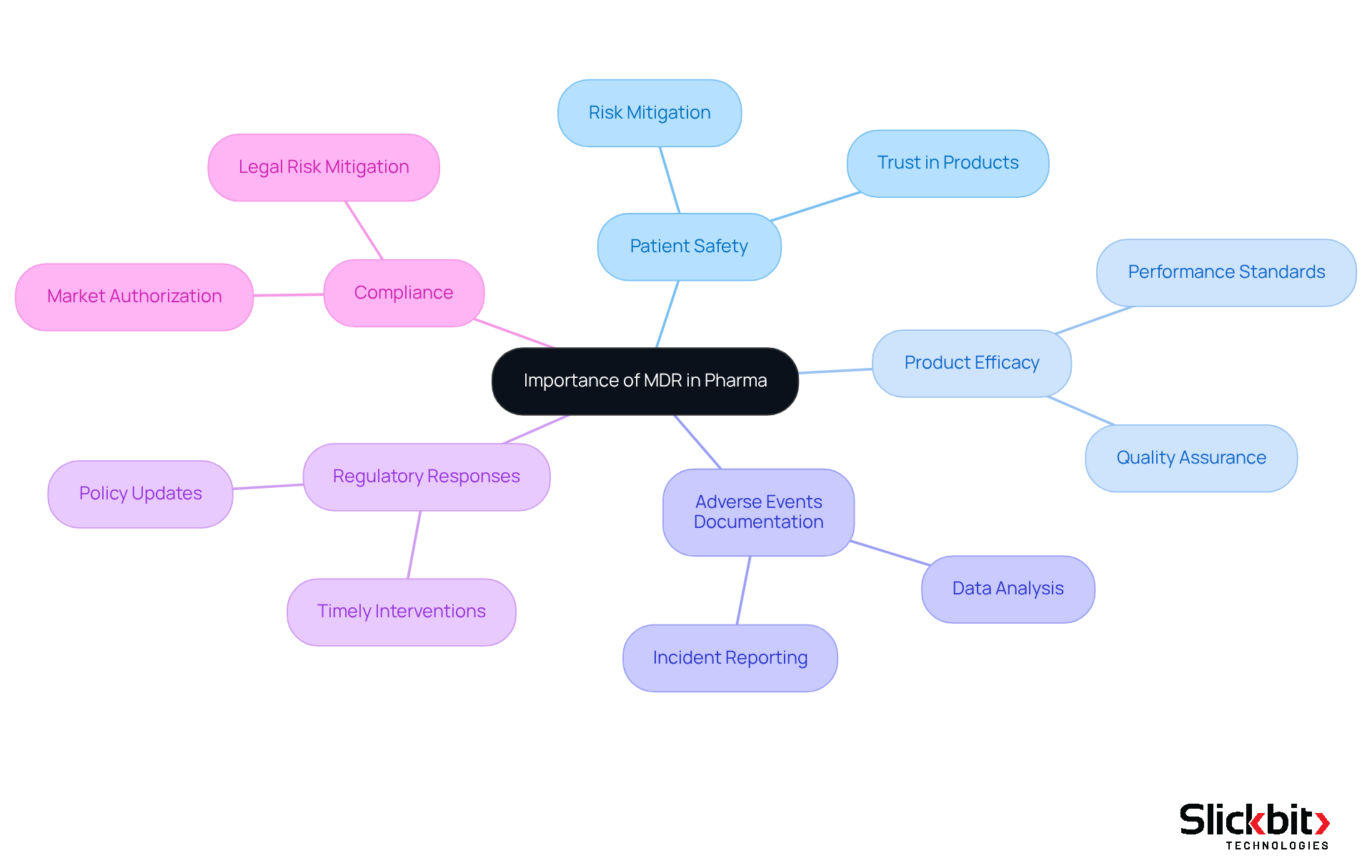
MDR médical plays a crucial role in the pharmaceutical industry, as it directly influences patient safety and product efficacy. By mandating the documentation of adverse events, MDR helps identify potential hazards associated with medical devices, enabling timely regulatory responses. This aspect is particularly vital in a sector where the stakes are high, and the consequences of equipment failures can be dire. Moreover, compliance with mdr médical regulations is essential for pharmaceutical companies to maintain their market authorization and mitigate legal risks, thereby establishing it as a fundamental aspect of responsible business practices.
Trace the Evolution of MDR Medical Reporting
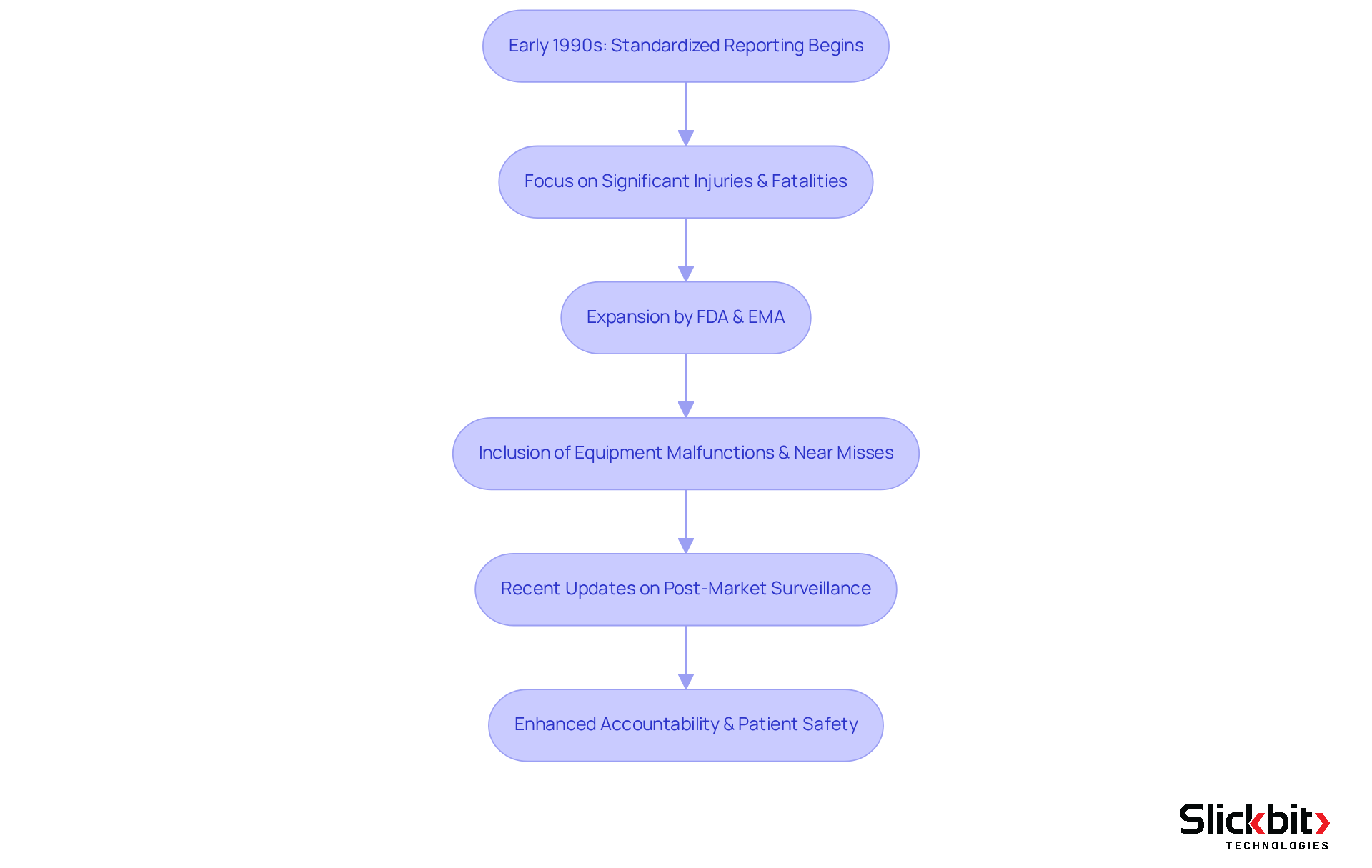
The evolution of mdr médical reporting began in the early 1990s, driven by the pressing need for standardized reporting of adverse events. Initially, the focus was to ensure that manufacturers reported significant injuries and fatalities associated with medical equipment. Over time, regulatory authorities, including the FDA in the United States and the European Medicines Agency in Europe, have expanded the scope of mdr médical to encompass a broader range of incidents, such as equipment malfunctions and near misses. Furthermore, recent updates underscore the importance of post-market surveillance and real-world evidence within the framework of mdr médical, indicating a shift towards a more proactive approach in monitoring safety. This evolution not only enhances the regulatory framework but also fosters greater accountability among manufacturers, ultimately prioritizing patient safety.
Outline Key Components and Requirements of MDR
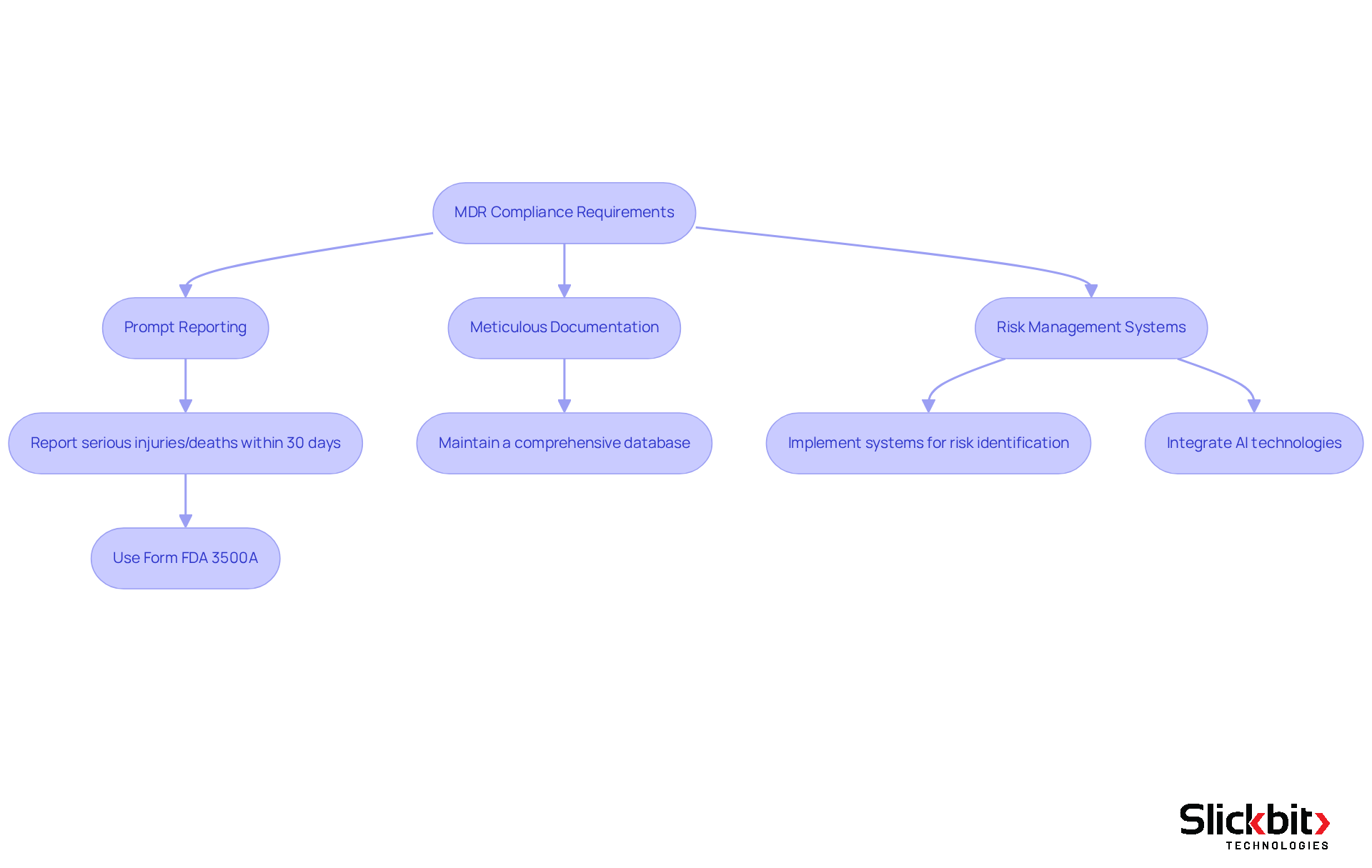
Key components of MDR médical reporting encompass stringent criteria for the prompt reporting of adverse occurrences, meticulous documentation of incidents, and the establishment of robust risk management systems. Manufacturers are required to report serious injuries or deaths associated with their devices within 30 calendar days of awareness, employing Form FDA 3500A for mandatory submissions. Furthermore, they must maintain a comprehensive database of reported incidents, ensuring that every device-related occurrence is documented and analyzed for performance insights.
As we approach 2025, compliance requirements for manufacturers will necessitate the implementation of effective risk management systems. Studies indicate that approximately 70% of manufacturers have already adopted such systems to fulfill MDR standards. These systems are crucial for identifying potential risks and ensuring that corrective actions are executed promptly. In addition, the integration of AI technologies can significantly enhance these initiatives by detecting trends in FDA inspections and streamlining the documentation process, ultimately leading to improved compliance outcomes.
Regular audits are imperative to verify adherence to regulations related to MDR médical, underscoring the necessity of ongoing monitoring and improvement. Training staff on MDR médical requirements is essential for fostering a culture of safety within organizations. This encompasses educating teams on the importance of accurate reporting and the procedures for documenting adverse events. By prioritizing these elements and incorporating AI tools for compliance insights, manufacturers can strengthen their compliance efforts and contribute to enhanced patient safety outcomes.
Conclusion
MDR médical reporting stands as a cornerstone in the realm of medical product safety, playing a pivotal role in ensuring that manufacturers and importers comply with regulatory obligations that ultimately protect patient well-being. By effectively documenting and communicating adverse events, this process not only fosters accountability among stakeholders but also enhances the overall integrity of medical devices in circulation.
The critical importance of MDR in the pharmaceutical sector is underscored by its significant influence on patient safety and product efficacy. Tracing the evolution of MDR reporting from its inception, we observe how regulatory frameworks have adapted to encompass a broader range of incidents and proactive monitoring. Essential components, such as stringent reporting criteria and robust risk management systems, are vital for manufacturers to maintain compliance and safeguard patient health.
Reflecting on the insights presented, it is evident that the future of MDR médical reporting is closely linked with technological advancements, particularly the integration of AI. As the landscape continues to evolve, a commitment to rigorous compliance and continuous improvement will be crucial. Engaging in these practices not only fulfills regulatory requirements but also reinforces a culture of safety that is indispensable in the healthcare industry. Embracing these principles will ensure that patient safety remains at the forefront of medical device innovation and regulation.
Frequently Asked Questions
What is MDR medical reporting?
MDR medical reporting, also known as medical product reporting, is a regulatory obligation for manufacturers and importers of medical products to report adverse events and product issues to regulatory authorities.
Why is MDR medical reporting important?
It is essential for ensuring patient safety and maintaining the integrity of medical instruments that comply with MDR regulations in the market.
What activities are involved in MDR medical reporting?
MDR encompasses activities such as the collection, analysis, and communication of information related to equipment performance and safety.
How does MDR medical reporting facilitate interventions?
By reporting adverse events and product issues, MDR medical reporting allows for timely interventions when problems arise.
How can AI technology enhance the MDR medical reporting process?
The integration of AI technology can improve the process by identifying trends in compliance and systemic risks, thereby enhancing the efficiency and effectiveness of MDR documentation.




