Overview
This article delineates the concept of Good Manufacturing Practices (GMP) and underscores their critical importance for managers involved in pharmaceutical research and development. GMP comprises essential systems and guidelines that ensure drug safety, quality, and regulatory compliance. Adhering to these practices not only mitigates risks associated with pharmaceutical production but also significantly enhances operational efficiency and assures public health. Consequently, understanding and implementing GMP is imperative for industry professionals committed to advancing drug safety and efficacy.
Introduction
The pharmaceutical industry is pivotal in safeguarding public health, where the integrity of drug manufacturing is of utmost importance. Good Manufacturing Practices (GMP) form the foundation of this sector, guaranteeing that each product is not only effective but also safe for consumption. As the regulatory landscape continues to evolve, it is essential for pharmaceutical R&D managers to grasp the complexities of GMP compliance while upholding superior quality standards. Organizations encounter various challenges in implementing these practices, and it is imperative to explore how they can leverage technology to enhance their operations and protect public health.
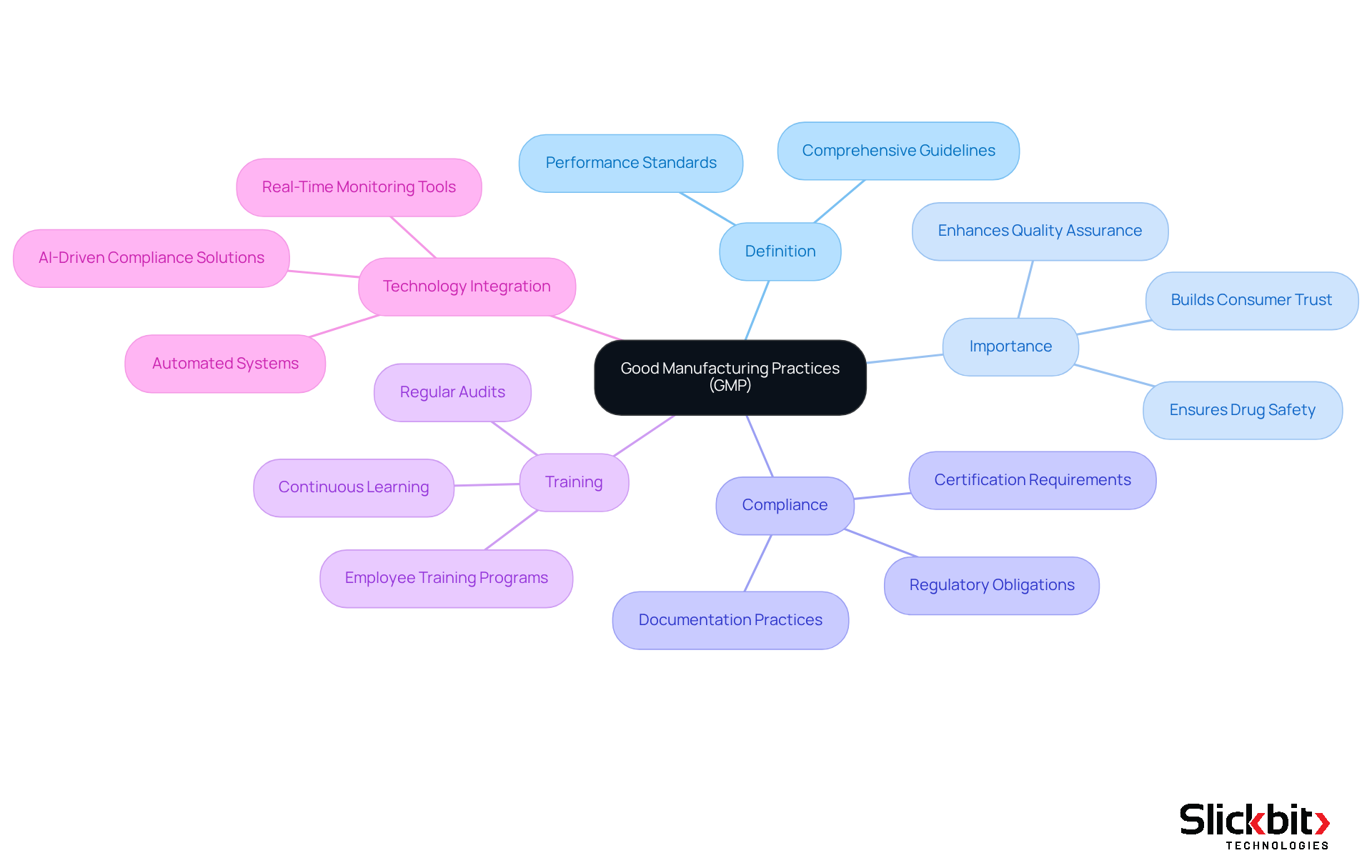
Define Good Manufacturing Practices (GMP)
The gmp definition encompasses essential systems and guidelines that ensure pharmaceutical products are produced and regulated consistently according to rigorous performance standards. These practices are crucial for ensuring that drugs are safe, effective, and of the highest quality. The definition of GMP encompasses every aspect of production, including the selection of raw materials, the facilities and equipment utilized, as well as the training and hygiene of personnel. Complying with the gmp definition is not merely a regulatory obligation; it reflects a profound commitment to excellence that safeguards public health.
In 2025, the gmp definition plays a heightened role in drug safety and quality due to evolving regulatory frameworks and increasing consumer expectations. Companies that adhere to the gmp definition not only mitigate the risks of product recalls and legal penalties but also bolster their market reputation and consumer trust. Organizations that meet the gmp definition certification requirements can avert significant legal ramifications, including recalls and business interruptions, thereby underscoring the critical nature of these practices in maintaining high-quality benchmarks.
The successful implementation of the GMP definition in drug manufacturing necessitates regular training sessions for employees to ensure they comprehend and apply GMP requirements in their daily operations. Continuous learning and adaptation to the latest guidelines, including the gmp definition, are vital for maintaining compliance and avoiding costly errors. Furthermore, the integration of automated systems, such as Slickbit's Vault Redact for secure content redaction and Trend 483 for compliance insights, can enhance control processes. These solutions minimize human errors and ensure consistent adherence to the GMP definition, which ultimately leads to improved safety and effectiveness of the products.
Contextualize GMP in Pharmaceutical R&D
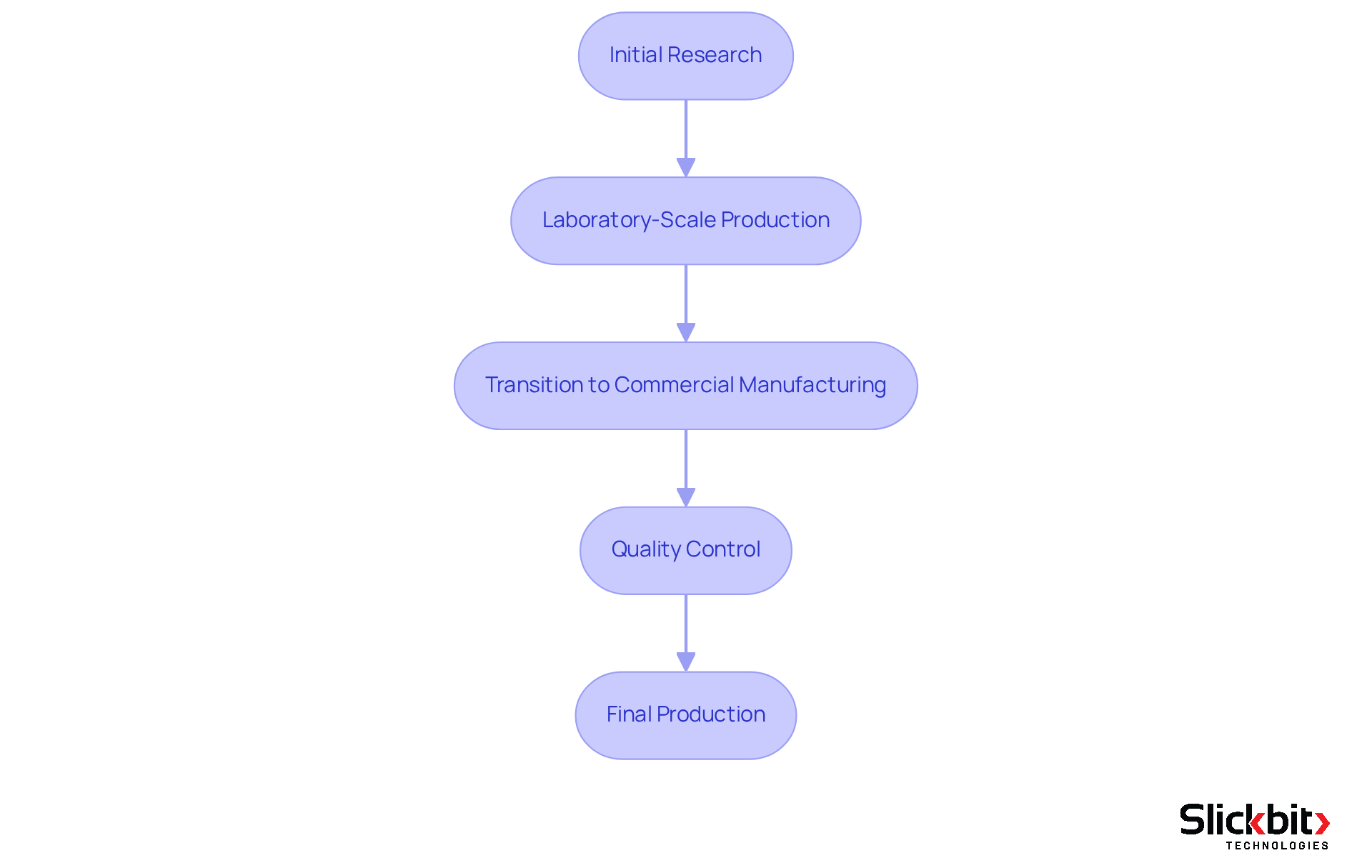
In the realm of pharmaceutical research and development, the GMP definition represents a vital framework that governs drug manufacturing processes. These practices are essential to ensuring that every phase of drug development, from initial research to final production, adheres to rigorous quality standards. This adherence becomes particularly critical during the transition from laboratory-scale production to commercial manufacturing, as it safeguards the integrity and safety of the product.
By integrating GMP principles into R&D processes, organizations can effectively mitigate risks associated with contamination, mix-ups, and errors. Such a proactive strategy not only enhances safety but also fosters greater efficiency in development timelines.
Experts underscore that a robust GMP definition is essential for maintaining compliance and ensuring that pharmaceutical products meet the highest standards of excellence, ultimately benefiting both consumers and the industry at large.
Trace the Historical Development of GMP
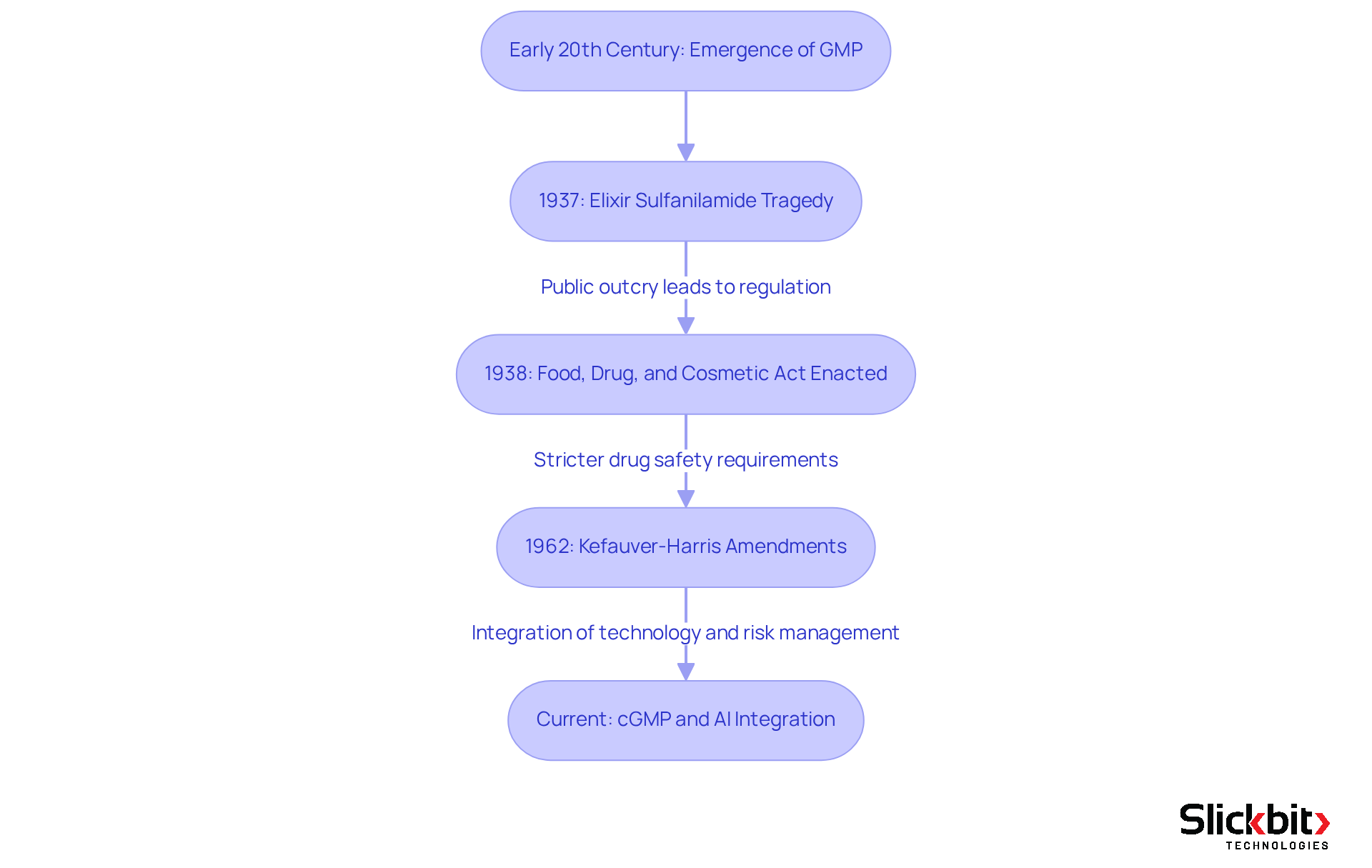
The gmp definition emerged in the early 20th century, driven by an urgent need for assurance within the pharmaceutical industry. A significant turning point occurred with the enactment of the Food, Drug, and Cosmetic Act in 1938, which empowered the FDA to regulate drug manufacturing processes in response to public health concerns, particularly highlighted by the Elixir Sulfanilamide tragedy in 1937 that led to over 100 fatalities.
Since that pivotal moment, GMP regulations have undergone substantial evolution, influenced by public health crises and technological advancements. The current Good Manufacturing Practices (cGMP) enforced by the FDA focus on risk management and continuous improvement, ensuring that pharmaceutical products consistently adhere to stringent safety and efficacy standards.
Experts observe that the evolution of GMP has frequently been reactive, with many requirements instituted following tragic incidents. For example, the Kefauver-Harris Amendments of 1962 mandated that drug manufacturers demonstrate both safety and efficacy prior to marketing, marking a shift towards more rigorous oversight. The FDA’s ongoing adjustments to GMP regulations reflect a steadfast commitment to enhancing consumer protection amid an ever-evolving landscape.
In today’s context, the GMP definition transcends mere regulatory obligation; it represents a vital component of assurance within the pharmaceutical sector. The FDA’s comprehensive approach to GMP encompasses meticulous documentation, qualified personnel, and extensive control procedures, enabling manufacturers to effectively mitigate risks associated with drug production. Furthermore, the integration of AI technologies, such as Trend 483, empowers companies to identify trends in systemic risks and compliance issues derived from FDA 483s, thereby enhancing operational efficiency and providing deeper insights into compliance practices. This evolution underscores the critical importance of upholding high standards in pharmaceutical manufacturing, ultimately safeguarding public health.
Identify Key Components and Principles of GMP
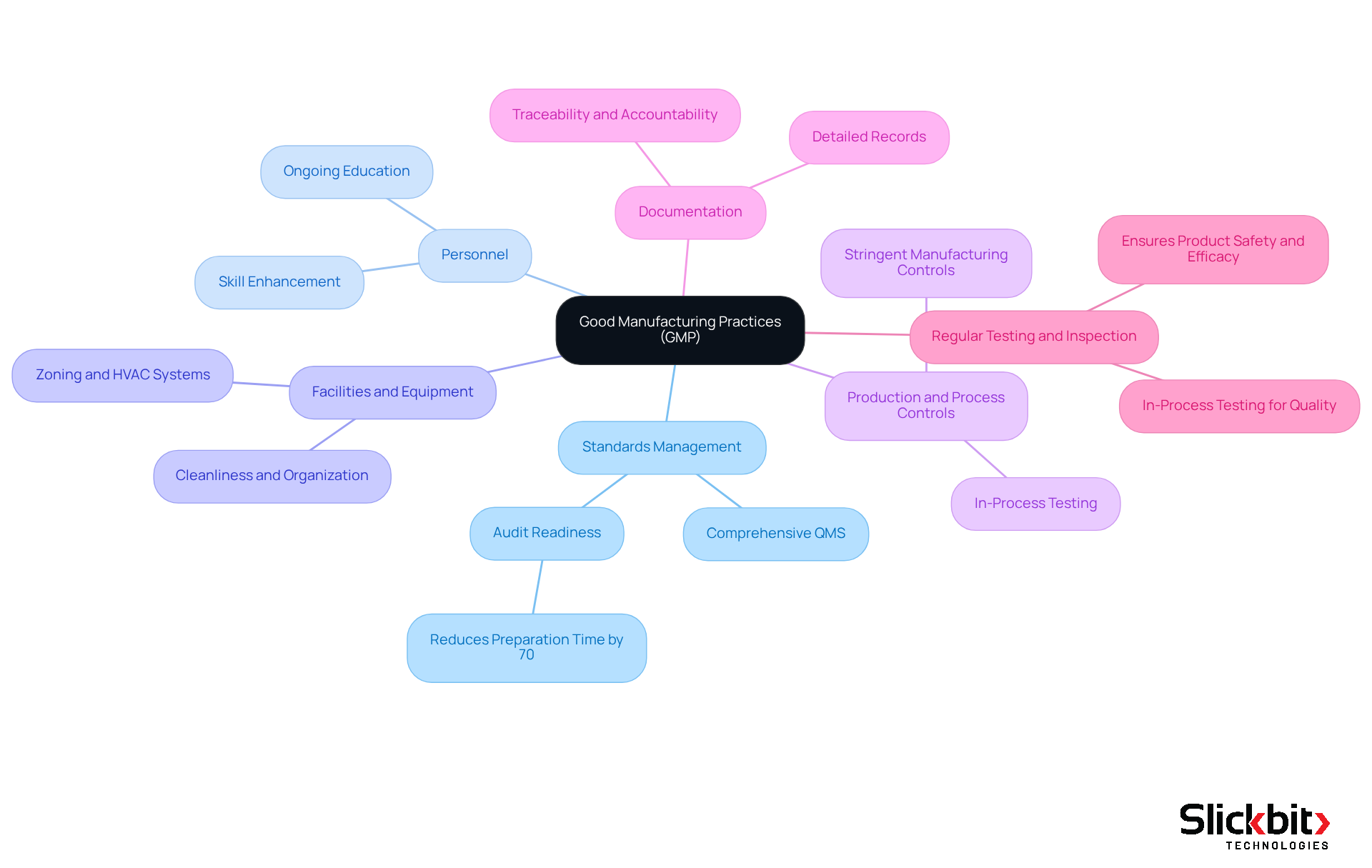
The gmp definition includes key components that are critical for ensuring compliance and operational excellence in the pharmaceutical industry.
-
Standards Management: Establishing a comprehensive standards management system (QMS) that integrates all production aspects is essential. A robust QMS not only streamlines processes but also enhances audit readiness, reducing preparation time by up to 70%. This integration fosters a culture of compliance and operational efficiency.
-
Personnel: Ensuring that staff are adequately trained and qualified is crucial. Efficient staff training initiatives should emphasize ongoing education and skill enhancement, promoting a culture of excellence and responsibility within the organization. This commitment to personnel development directly correlates with improved operational outcomes.
-
Facilities and Equipment: Maintaining clean, well-organized facilities and equipment is vital to prevent contamination. To uphold the GMP definition, proper zoning, HVAC systems, and sanitation practices are necessary. These measures not only protect product integrity but also enhance overall operational efficiency.
-
Production and Process Controls: Implementing stringent controls over manufacturing processes ensures consistency and excellence. This encompasses outlining and validating in-process controls and testing to prevent contamination and ensure item integrity. By prioritizing these controls, companies can significantly reduce the risk of product defects.
-
Documentation: Keeping detailed records of all manufacturing processes is essential for traceability and accountability. Documentation control ensures that records are accurate, accessible, and unaltered, reinforcing compliance with regulatory requirements. The ability to search and filter FDA 483s for deeper insights can further enhance documentation practices, ensuring that companies remain proactive in their compliance efforts.
-
Regular testing and inspection of items are necessary to ensure they meet established standards. This includes in-process testing for identity, strength, quality, and purity, which is critical for maintaining product safety and efficacy. By adhering to the gmp definition, pharmaceutical companies can significantly enhance their operational efficiency and ensure compliance with regulatory requirements, ultimately leading to improved patient outcomes.
Conclusion
Good Manufacturing Practices (GMP) are fundamental to the pharmaceutical industry, ensuring that products are consistently produced and controlled to quality standards that protect public health. This comprehensive definition of GMP encompasses every aspect of drug production, from the selection of raw materials to the training of personnel, reflecting a commitment to excellence that is both regulatory and ethical.
The importance of adhering to GMP principles is paramount, especially in the realm of pharmaceutical research and development. A robust quality management system, continuous employee training, and the integration of advanced technologies are essential to enhance compliance and operational efficiency. Historical developments demonstrate how GMP regulations have evolved in response to public health crises, reinforcing their critical role in safeguarding drug safety and efficacy.
Ultimately, the focus on GMP extends beyond mere compliance; it embodies a proactive approach that fosters trust and reliability in the pharmaceutical sector. By embracing these practices, organizations not only mitigate risks associated with drug manufacturing but also contribute to a culture of safety and quality that benefits consumers and the industry alike. The imperative is clear: prioritizing GMP is essential for any pharmaceutical entity aiming to thrive in an increasingly regulated and competitive landscape.
Frequently Asked Questions
What are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are essential systems and guidelines that ensure pharmaceutical products are produced and regulated consistently according to rigorous performance standards, ensuring drugs are safe, effective, and of the highest quality.
What aspects of production does the GMP definition cover?
The GMP definition encompasses every aspect of production, including the selection of raw materials, the facilities and equipment used, and the training and hygiene of personnel.
Why is compliance with GMP important?
Compliance with GMP is crucial not only as a regulatory obligation but also as a commitment to excellence that safeguards public health and mitigates risks of product recalls and legal penalties.
How does the GMP definition impact drug safety and quality in 2025?
In 2025, the GMP definition plays a heightened role in drug safety and quality due to evolving regulatory frameworks and increasing consumer expectations, helping companies enhance their market reputation and consumer trust.
What are the consequences of not adhering to the GMP definition?
Organizations that fail to meet GMP certification requirements may face significant legal ramifications, including product recalls and business interruptions.
What is necessary for the successful implementation of GMP in drug manufacturing?
Successful implementation of GMP requires regular training sessions for employees to ensure they understand and apply GMP requirements in their daily operations.
How can technology aid in maintaining GMP compliance?
The integration of automated systems, such as Slickbit's Vault Redact for secure content redaction and Trend 483 for compliance insights, can enhance control processes, minimize human errors, and ensure consistent adherence to GMP.
What is the ultimate goal of adhering to the GMP definition?
The ultimate goal of adhering to the GMP definition is to improve the safety and effectiveness of pharmaceutical products.




