Overview
The full form of DMR is Device Master Record, a crucial document that consolidates all specifications, procedures, and quality assurance measures necessary for the production of medical devices. Understanding the significance of the DMR is essential for compliance with regulatory standards. It ensures product safety and quality throughout the manufacturing process. Manufacturers are required to maintain comprehensive documentation, which includes:
- Device specifications
- Production processes
- Quality assurance protocols
Consequently, the DMR serves as a foundational element in the medical device industry, safeguarding both compliance and product integrity.
Introduction
Understanding the intricacies of the Device Master Record (DMR) is essential for stakeholders in the medical device industry. This vital document consolidates the specifications and procedures necessary for manufacturing, while also playing a pivotal role in ensuring compliance with stringent regulatory standards.
As the landscape of medical device regulation evolves, manufacturers face the ongoing challenge of navigating these complexities while maintaining product safety and quality. By exploring the DMR's definition, key components, and its significance in regulatory practices, we uncover the critical framework that underpins successful medical device production.
Define Device Master Record (DMR)
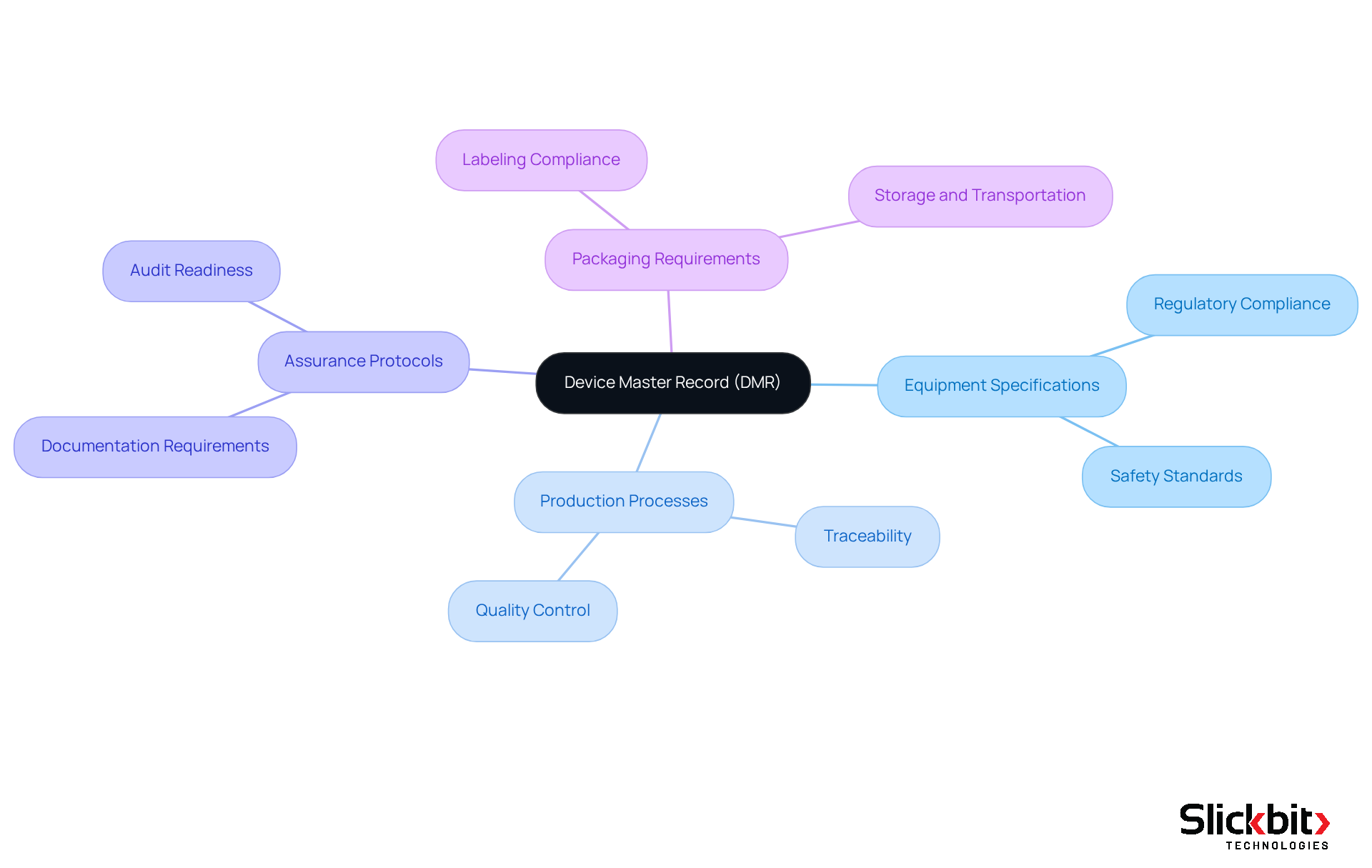
The DMR full form, or Device Master Record, is an essential document that consolidates all specifications, procedures, and assurance measures necessary for the production of a specific medical device. It is pivotal in ensuring that equipment is manufactured in accordance with regulatory standards and operates as intended. The DMR encompasses vital information, including:
- Equipment specifications
- Production processes
- Assurance protocols
- Packaging requirements
This comprehensive collection not only facilitates compliance with standards set by organizations such as the FDA but also serves as a foundation for ensuring product excellence throughout its lifecycle.
In fact, a significant percentage of medical device manufacturers—over 70%—report that they maintain a DMR as part of their management systems. This statistic highlights the critical importance of the DMR full form in the industry, as it ensures traceability and accountability in the manufacturing process. Manufacturers are mandated to retain records for a minimum of two years from the date of release for commercial distribution, further underscoring the DMR's essential role in compliance and product safety. As emphasized by industry experts, the DMR full form is vital for preventing product failures and ensuring user safety, aligning with the FDA's focus on stringent documentation and quality control.
Moreover, the successful implementation of the DMR is evident in various life sciences applications, where companies have leveraged it to streamline production and enhance compliance. For instance, manufacturers have integrated DMRs with electronic Quality Management Systems (eQMS) to facilitate real-time updates and ensure that all documentation remains audit-ready. This integration not only boosts operational efficiency but also assists in meeting evolving compliance demands, such as the forthcoming Quality Management System Regulation (QMSR), which is set to take effect in 2026.
Contextualize DMR in Medical Device Regulation
In the medical equipment sector, the Device Master Record (DMR) is essential for ensuring that products are manufactured consistently and in compliance with regulatory standards. Regulatory agencies, including the FDA, mandate that manufacturers maintain a DMR as part of their management system, underscoring the critical need for medical devices to be safe and effective for public use.
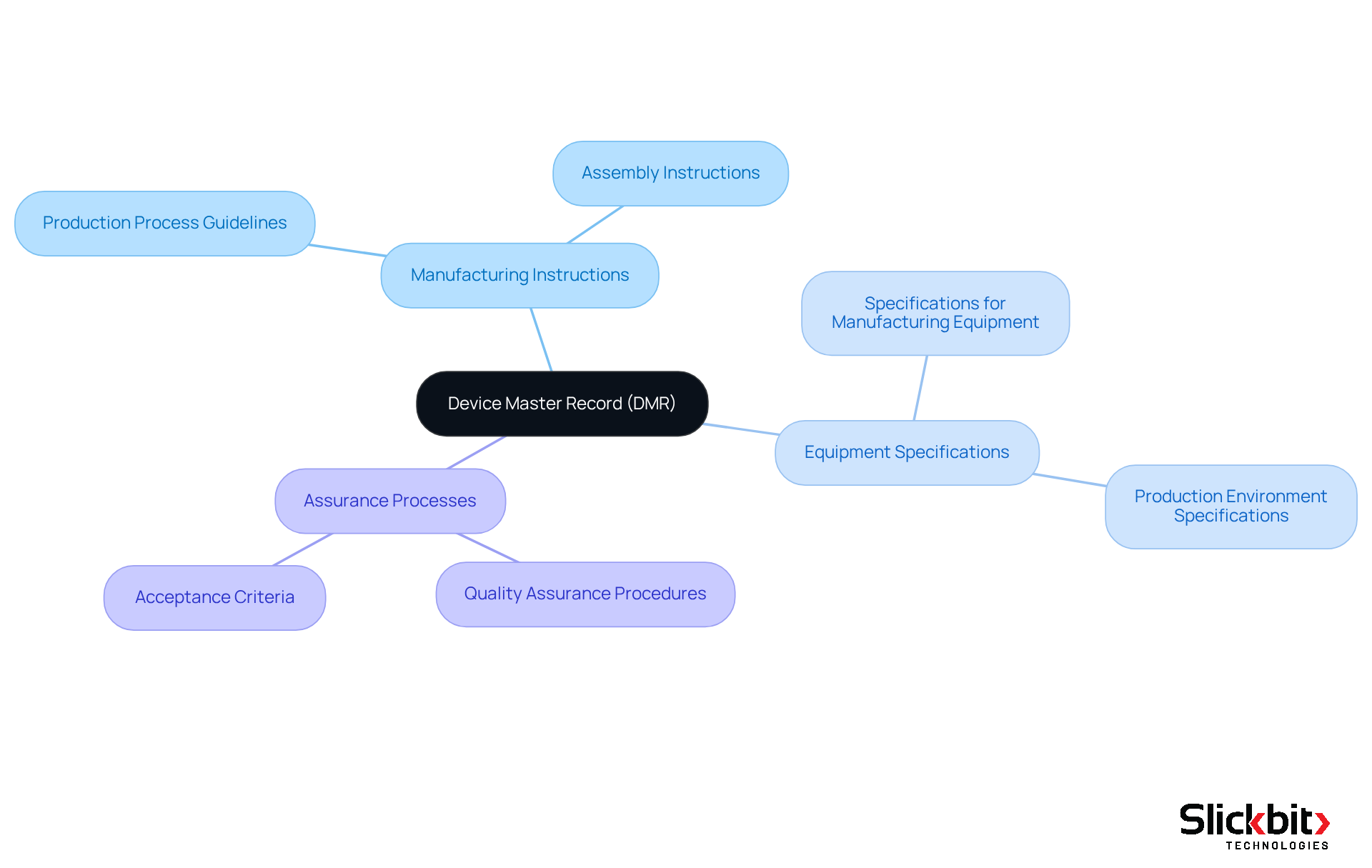
The DMR acts not only as a comprehensive reference for manufacturers but also as a vital documentation tool for regulators during inspections and audits. It encompasses crucial information such as:
- Manufacturing instructions
- Equipment specifications
- Assurance processes
Thereby facilitating adherence to FDA standards. This systematic approach to documentation is indispensable for successfully navigating compliance audits and ensuring ongoing adherence to safety and quality requirements.
Trace the Evolution of DMR in Regulatory Practices
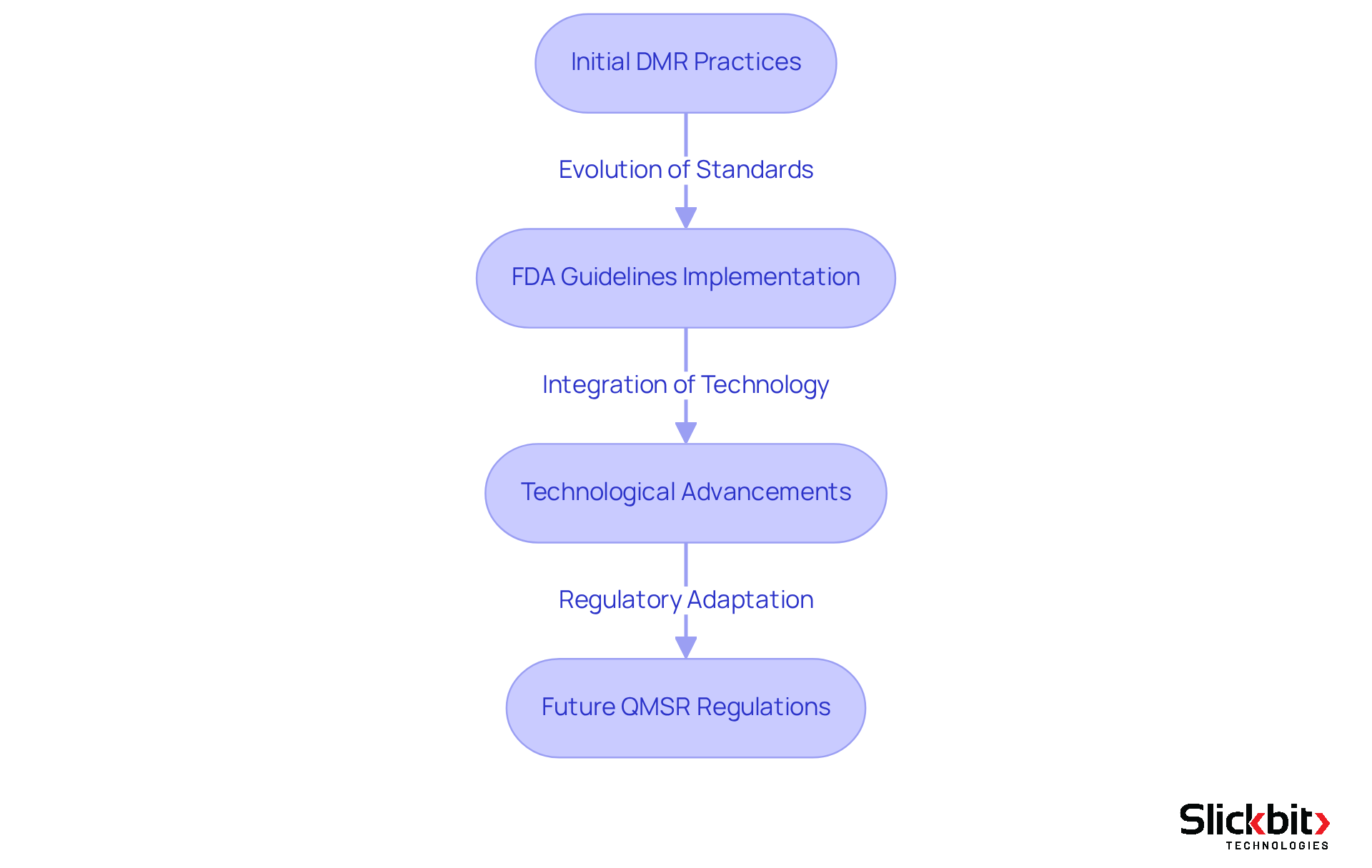
The development of the Device Master Record, or DMR full form, has been significant since its inception within the medical equipment oversight landscape. Initially, producers faced limited paperwork demands, which often resulted in discrepancies in product standards and safety. As the medical equipment sector expanded and oversight intensified, the FDA implemented stricter guidelines, establishing the DMR full form as a crucial component of the quality management system. This transformation was driven by the necessity for improved accountability and traceability in device production.
In recent years, the DMR full form has been further refined to align with technological advancements and evolving compliance expectations. For example, the FDA's forthcoming Quality Management System Regulation (QMSR), effective February 2, 2026, will incorporate ISO 13485:2016 standards, requiring manufacturers to adapt their DMR practices accordingly. This regulatory evolution underscores the importance of maintaining comprehensive documentation that includes design specifications, manufacturing instructions, and assurance procedures.
Statistics indicate that the medical equipment sector is projected to reach a market size of $678.88 billion by 2025, reflecting the growing demand for innovative and compliant products. This increase in market size emphasizes the critical need for manufacturers to enhance their practices related to the DMR full form to ensure compliance and effectively meet patient safety standards.
Case studies illuminate best practices in DMR documentation, highlighting the use of standardized templates, robust change control, and ongoing staff training, which are essential for understanding the DMR full form. These practices not only bolster compliance but also streamline operations, ultimately leading to improved product standards and safety. As articulated by the FDA, "Each organization will establish a QMS tailored to its needs concerning product safety and effectiveness, including in relation to records and documentation." The continuous commitment to refining DMR processes, referring to the DMR full form, demonstrates the industry's dedication to ongoing improvement and the protection of public health.
Identify Key Components and Requirements of DMR
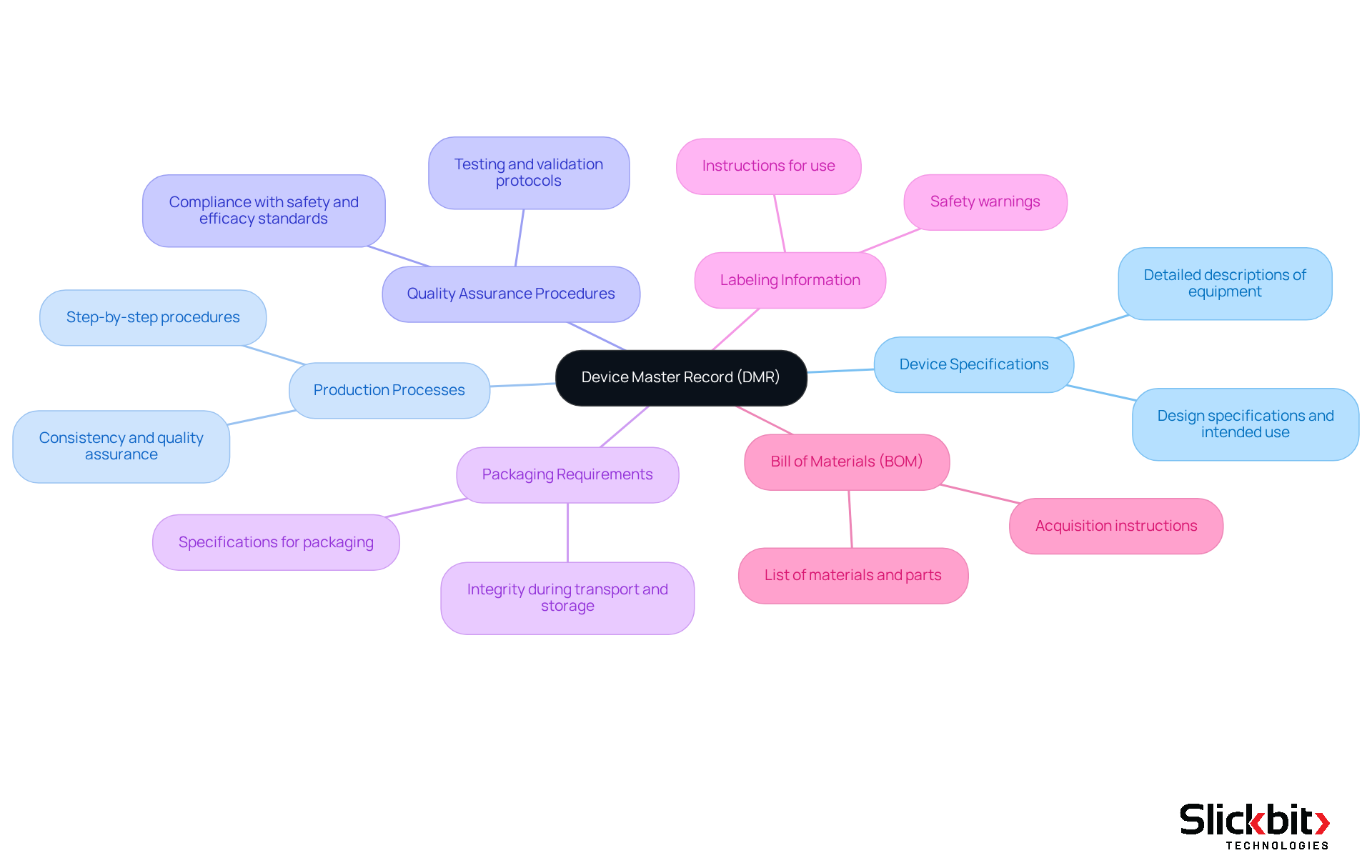
A comprehensive Device Master Record (DMR full form) must include several critical components to comply with regulatory standards and support effective management of excellence. These components typically encompass:
- Device Specifications: Detailed descriptions of the equipment, covering design specifications and intended use, ensure clarity in what the equipment is meant to achieve.
- Production Processes: Step-by-step procedures for creating the product are outlined to guarantee consistency and quality throughout the production lifecycle.
- Quality Assurance Procedures: Protocols for testing and validating the equipment are essential to ensure it meets established safety and efficacy standards, as mandated by regulatory bodies.
- Packaging Requirements: Specifications detailing how the apparatus should be packaged are crucial for maintaining integrity during transport and storage, preventing damage or contamination.
- Labeling Information: Comprehensive details on how the equipment should be labeled, encompassing instructions for use and necessary safety warnings, inform users effectively.
- Bill of Materials (BOM): The DMR must include or reference the required Bill of Materials, which lists all materials and parts needed for manufacturing the device.
By incorporating these components, manufacturers can ensure that their DMR full form not only meets compliance requirements, including those outlined in FDA regulations (21 CFR Part 820.181), but also enhances the overall quality management system. Furthermore, the incorporation of AI tools, like Trend 483, can streamline compliance by identifying trends in systemic risks and repeated violations from FDA inspections, thus facilitating smoother audits and oversight inspections. Proper documentation management is critical; poorly managed documentation can lead to audit findings and compliance penalties. Additionally, the DMR full form is informed by the Design History File (DHF), which documents the design journey of the device, ensuring that all specifications align with regulatory expectations. This comprehensive approach not only supports compliance but also enhances operational efficiency.
Conclusion
The Device Master Record (DMR) stands as a cornerstone of medical device manufacturing, serving as a comprehensive document that consolidates critical specifications, processes, and quality assurance measures. By ensuring adherence to regulatory standards, the DMR plays a vital role in maintaining product safety and efficacy, ultimately protecting public health. Understanding the full form of the DMR and its implications is essential for manufacturers aiming to streamline their operations and enhance compliance.
Throughout this article, key insights have been highlighted regarding the DMR's role in regulatory frameworks, particularly its necessity in meeting FDA requirements. The discussion has covered the essential components of a DMR, including device specifications, production processes, and quality assurance protocols. Furthermore, the evolution of DMR regulations demonstrates the increasing complexity and importance of maintaining thorough documentation in the face of advancing technology and regulatory expectations.
In light of the growing medical device market and its projected expansion, manufacturers must prioritize the implementation of robust DMR practices. By embracing standardized documentation, ongoing training, and the integration of modern technologies, organizations can ensure compliance while fostering an environment of continuous improvement. This commitment to excellence in DMR management transcends regulatory obligation; it represents a crucial step toward ensuring the safety and effectiveness of medical devices for all users.
Frequently Asked Questions
What does DMR stand for?
DMR stands for Device Master Record.
What is the purpose of a Device Master Record (DMR)?
The DMR is an essential document that consolidates all specifications, procedures, and assurance measures necessary for the production of a specific medical device, ensuring compliance with regulatory standards and proper operation.
What information is included in a DMR?
A DMR includes equipment specifications, production processes, assurance protocols, and packaging requirements.
Why is maintaining a DMR important for medical device manufacturers?
Maintaining a DMR is critical for compliance with standards set by organizations like the FDA, ensuring traceability, accountability, and product excellence throughout the device's lifecycle.
How long are manufacturers required to retain DMR records?
Manufacturers are mandated to retain DMR records for a minimum of two years from the date of release for commercial distribution.
What role does the DMR play in product safety?
The DMR is vital for preventing product failures and ensuring user safety, aligning with the FDA's focus on stringent documentation and quality control.
How has the DMR been integrated into modern manufacturing practices?
Manufacturers have integrated DMRs with electronic Quality Management Systems (eQMS) to facilitate real-time updates and maintain audit-ready documentation.
What benefits does integrating DMRs with eQMS provide?
This integration boosts operational efficiency and helps meet evolving compliance demands, such as the upcoming Quality Management System Regulation (QMSR) set to take effect in 2026.




