Overview
The meaning of CMC in the pharmaceutical sector—standing for Chemistry, Manufacturing, and Controls—plays a pivotal role in ensuring the consistent quality and safety of pharmaceutical products throughout their development and production processes. Effective CMC practices are not merely a regulatory requirement; they are essential for minimizing risks associated with drug development. This is evidenced by the significant impact that CMC-related issues can have on Investigational New Drug filings, ultimately influencing the overall success of medications in the market. Therefore, prioritizing robust CMC practices is crucial for pharmaceutical companies aiming to enhance product reliability and market performance.
Introduction
Understanding the intricacies of Chemistry, Manufacturing, and Controls (CMC) is essential for anyone navigating the complex landscape of pharmaceuticals. This critical framework not only governs the development and production of medications but also plays a pivotal role in ensuring their safety, efficacy, and regulatory compliance.
As the pharmaceutical industry confronts increasing challenges—such as stringent regulations and the demand for innovative manufacturing practices—the significance of robust CMC processes becomes even more pronounced.
Consequently, how can pharmaceutical companies effectively leverage CMC to mitigate risks and enhance drug development success in an ever-evolving market?
Define CMC: Meaning and Importance in Pharmaceuticals
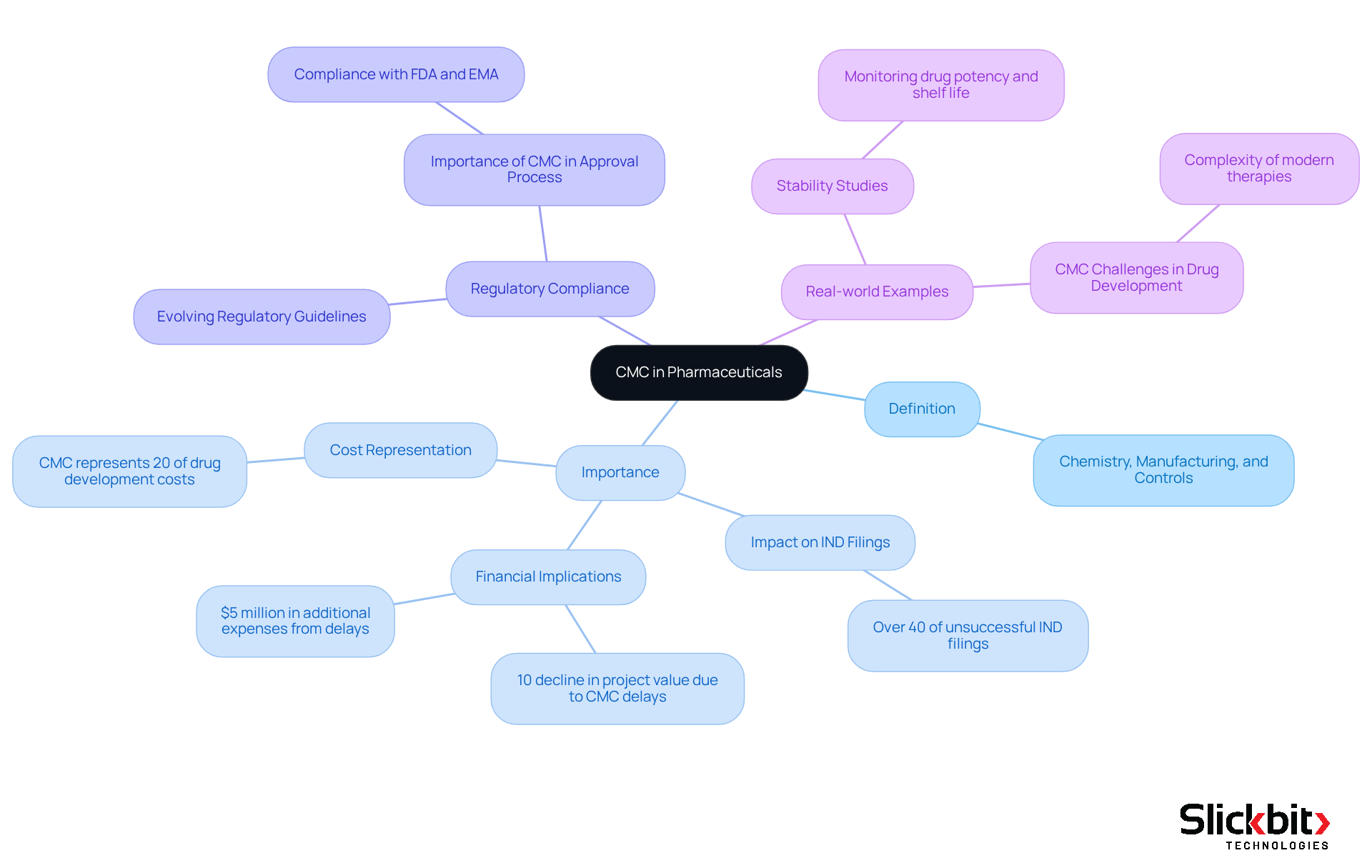
The CMC meaning in pharma, which refers to Chemistry, Manufacturing, and Controls, is fundamental to the development and production of pharmaceutical products. Its significance is paramount, as understanding CMC meaning in pharma ensures that medications are produced consistently, adhere to stringent quality standards, and comply with regulatory requirements. Notably, the cmc meaning in pharma highlights that CMC-related issues contribute to over 40% of unsuccessful Investigational New Drug (IND) filings, emphasizing the critical need for robust CMC practices throughout the drug development lifecycle.
Understanding the CMC meaning in pharma is vital in safeguarding the efficacy and safety of pharmaceutical products, which is a necessity in the highly regulated life sciences sector. By establishing rigorous criteria for chemical composition, production methods, and quality assurance, CMC guarantees that patients receive safe and effective medications. For example, a monoclonal antibody project with peak year sales potential exceeding $1 billion could experience a 10% decline in value due to a one-year CMC-related delay, underscoring the financial ramifications of CMC compliance.
Real-world examples further underscore the importance of CMC processes. Stability studies, meticulously designed and analyzed by CMC statisticians, monitor how environmental factors affect a medication's potency and shelf life, ensuring that products remain safe and effective throughout their lifecycle. Furthermore, efficient management of CMC elements can prevent unnecessary delays in product development, which is crucial as the complexity of pharmaceutical modalities increases.
In conclusion, the CMC meaning in pharma is not merely a regulatory requirement; it is a foundational element that guarantees the quality and safety of pharmaceutical products. This commitment ultimately protects patient health and enhances the credibility of the pharmaceutical industry.
Trace the Evolution of CMC in Drug Development
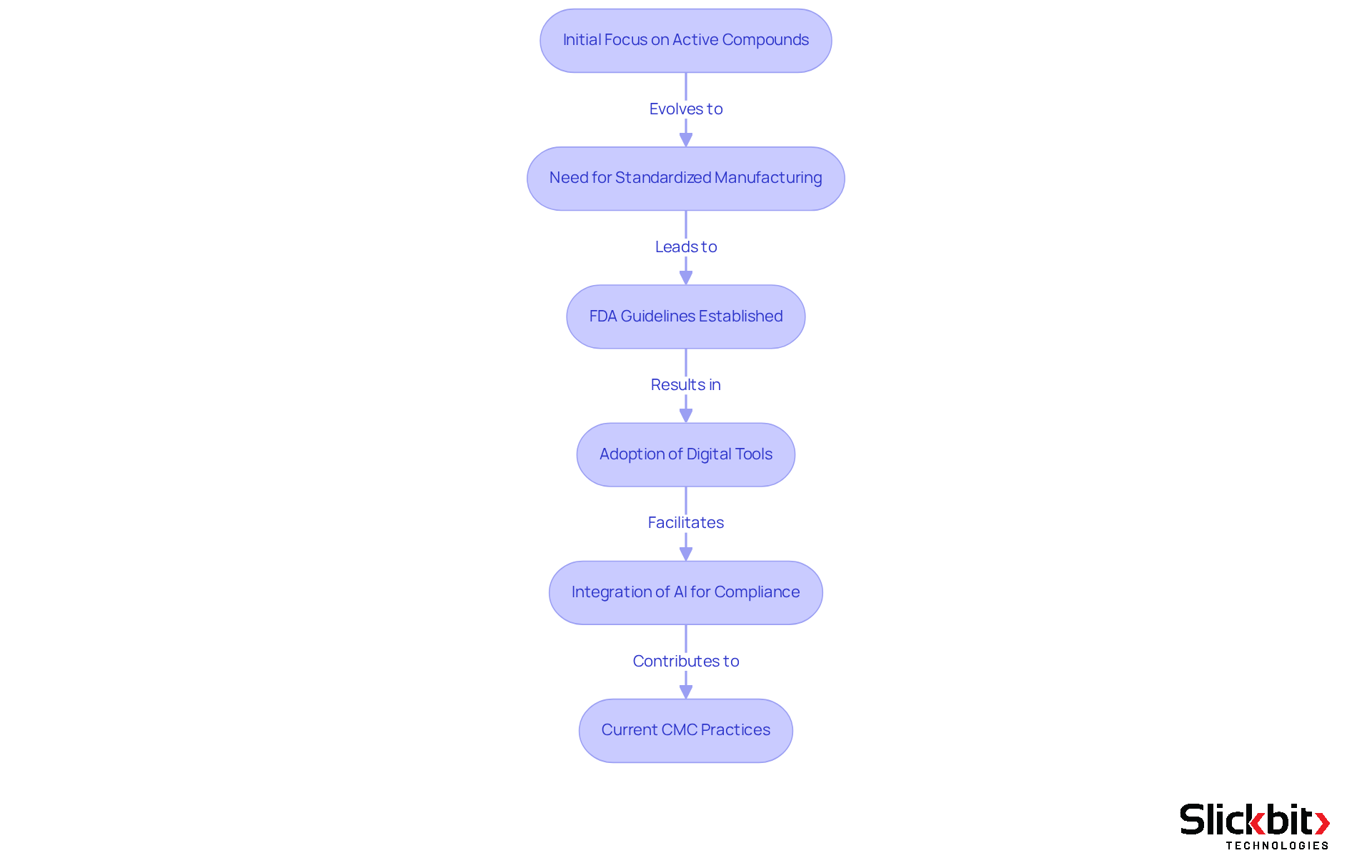
The evolution of Chemistry, Manufacturing, and Controls (CMC) in medication development illustrates the cmc meaning in pharma and marks a significant transformation in the pharmaceutical industry. Initially, the primary focus was on discovering active compounds. However, as the industry advanced, the need for standardized manufacturing processes and robust quality assurance became increasingly clear. In response, oversight organizations such as the FDA established guidelines mandating rigorous CMC practices, highlighting the cmc meaning in pharma, to ensure the safety and effectiveness of medications.
Furthermore, technological advancements and heightened oversight have fostered the development of more sophisticated CMC strategies. These strategies encompass the integration of digital tools and data analytics, which significantly enhance compliance and operational efficiency. For instance, Slickbit's Trend 483 utilizes AI to identify trends in systemic risks and compliance from FDA 483s, enabling users to search, filter, and examine full 483s for deeper insights.
Today, the CMC meaning in pharma is recognized as a crucial element of the development lifecycle, influencing every phase from initial research to market approval. It ensures that products meet the highest standards of quality and safety. Additionally, advancements in omics technologies present both challenges and opportunities, compelling CMC practices to adapt to these emerging scientific developments. With forthcoming guidelines, such as the MHRA's rule on decentralized manufacturing, the landscape of CMC, or cmc meaning in pharma, continues to evolve, underscoring its vital significance within the pharmaceutical sector.
Identify Key Characteristics and Components of CMC
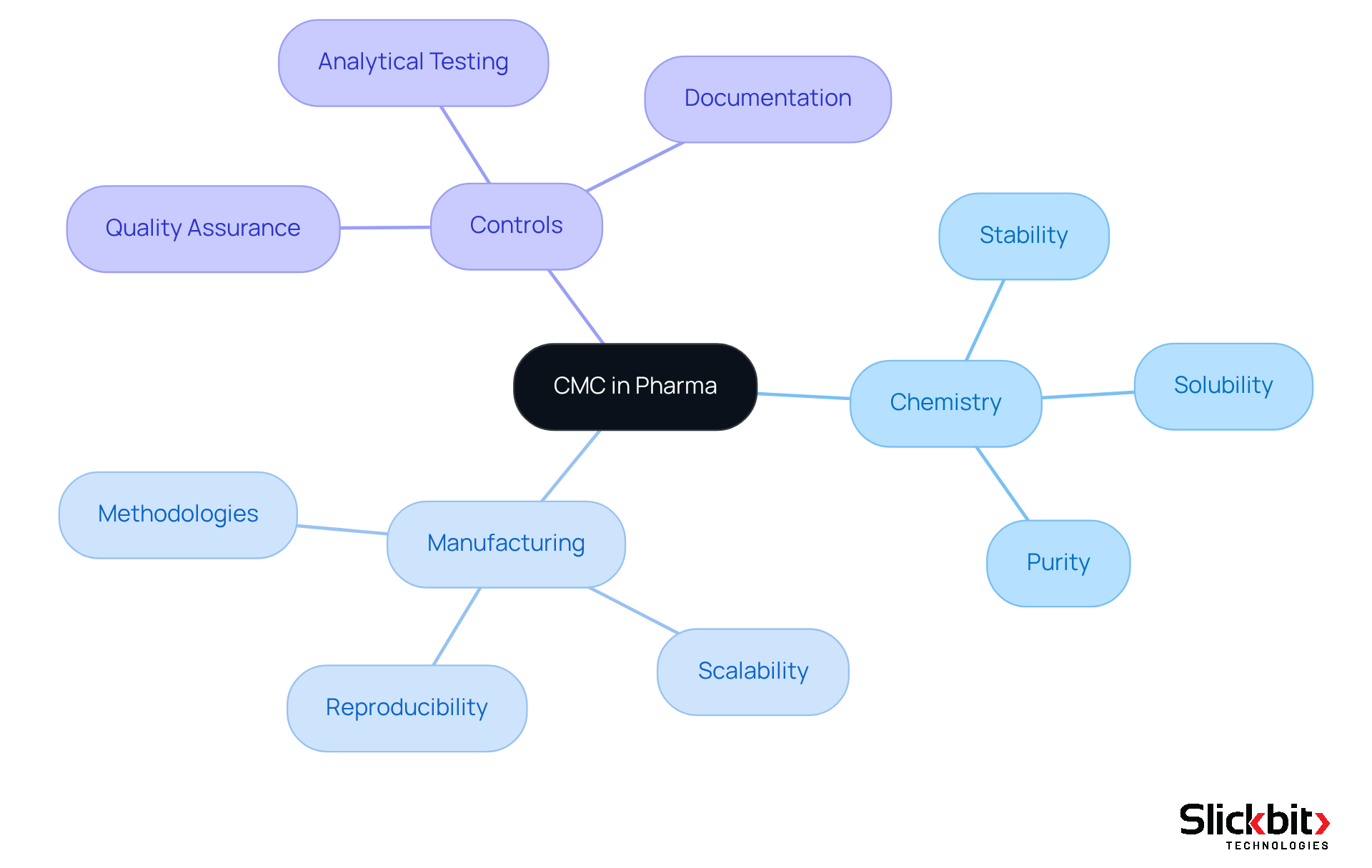
The essential characteristics of Chemistry, Manufacturing, and Controls (CMC), elaborating on cmc meaning in pharma, are anchored in three pivotal components: chemistry, manufacturing, and controls. The chemistry aspect underscores the significance of a compound's chemical properties—stability, solubility, and purity—critical for ensuring both efficacy and safety. Manufacturing highlights the methodologies employed in drug creation, focusing on scalability and reproducibility to meet compliance standards. Controls encompass the quality assurance measures that meticulously monitor and validate the manufacturing process, incorporating rigorous analytical testing and comprehensive documentation practices.
Producers are required to submit a CMC Common Data Set as part of the compliance submission procedure, which encompasses extensive information on chemical composition, manufacturing methods, and stability data. Notably, the medication approval timeline can extend from 10 to 15 years, reflecting the substantial time commitment inherent in CMC activities, ultimately highlighting the cmc meaning in pharma. CMC statisticians play a vital role in developing and evaluating stability studies, which are essential for compliance submissions. Collectively, these components establish a robust framework that ensures the quality and consistency of pharmaceutical products throughout their lifecycle.
Understanding the CMC meaning in pharma is paramount, as adherence to these standards is required by regulatory authorities such as the FDA and EMA to ensure that manufacturing methods are both reproducible and resilient. This compliance ultimately influences the success of medication approvals, highlighting the critical nature of CMC meaning in pharma within the pharmaceutical landscape.
Examine CMC's Role in Regulatory Compliance and Quality Assurance
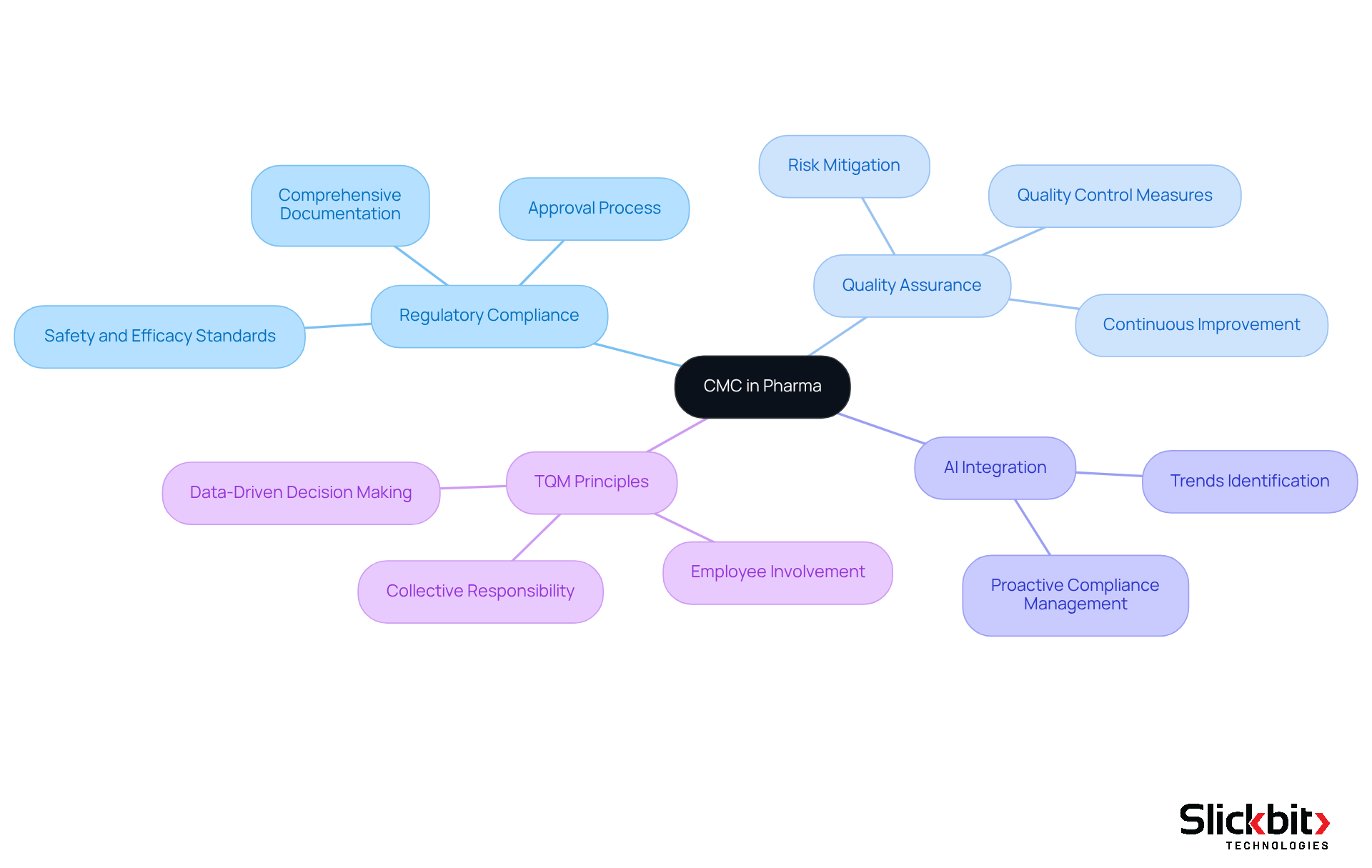
The CMC meaning in pharma refers to the essential components of regulatory compliance and quality assurance in the pharmaceutical industry. Regulatory bodies require comprehensive CMC documentation, reflecting the CMC meaning in pharma, as part of the medication approval process, ensuring that every aspect of pharmaceutical development adheres to established safety and efficacy standards. This requirement includes detailed descriptions of the substance's chemical characteristics, production methods, and quality assurance measures. By maintaining rigorous CMC practices, pharmaceutical companies can grasp the CMC meaning in pharma to mitigate risks associated with product recalls, compliance penalties, and reputational damage. Furthermore, understanding CMC meaning in pharma facilitates smoother interactions with regulatory bodies, ultimately expediting the approval timeline and enabling the timely introduction of safe and effective drugs to the market.
The integration of AI technologies can significantly enhance CMC practices by identifying trends in systemic risks and compliance issues, allowing companies to proactively address potential challenges. As W. Edwards Deming astutely observed, 'Eighty-five percent of the reasons for failure are deficiencies in the systems and process rather than the employee,' which underscores the critical importance of understanding CMC meaning in pharma practices. Additionally, the principles of Total Quality Management (TQM) highlight that quality is a collective responsibility across all departments, further emphasizing the integral role of the CMC meaning in pharma in cultivating a culture of quality assurance within the pharmaceutical sector.
Conclusion
Understanding the meaning of CMC in pharma is essential for the successful development and production of pharmaceutical products. This framework, which encompasses Chemistry, Manufacturing, and Controls, serves not only as a regulatory necessity but also as a cornerstone that ensures the quality and safety of medications. By adhering to robust CMC practices, pharmaceutical companies can navigate the complexities of drug development while safeguarding patient health.
The article highlights several key aspects of CMC, including:
- Its role in mitigating risks associated with drug approval failures
- The evolution of CMC practices in response to regulatory demands
- The integration of advanced technologies to enhance compliance and efficiency
Each of these elements underscores the critical nature of CMC in maintaining high standards of quality assurance and regulatory compliance, which are vital for the successful introduction of safe and effective drugs to the market.
In light of the significant challenges faced by the pharmaceutical industry, embracing the principles of CMC is imperative. Companies are encouraged to invest in robust CMC strategies and leverage technological advancements to streamline processes and ensure adherence to regulatory standards. By doing so, they not only enhance their operational efficiency but also contribute to the overall credibility and trustworthiness of the pharmaceutical sector.
Frequently Asked Questions
What does CMC stand for in pharmaceuticals?
CMC stands for Chemistry, Manufacturing, and Controls, which is essential for the development and production of pharmaceutical products.
Why is CMC important in the pharmaceutical industry?
CMC is important because it ensures that medications are produced consistently, meet quality standards, and comply with regulatory requirements, thereby safeguarding the efficacy and safety of pharmaceutical products.
What percentage of unsuccessful Investigational New Drug (IND) filings are attributed to CMC-related issues?
Over 40% of unsuccessful IND filings are attributed to CMC-related issues, highlighting the need for robust CMC practices.
How can CMC compliance impact the financial value of pharmaceutical projects?
For instance, a monoclonal antibody project with peak year sales potential exceeding $1 billion could experience a 10% decline in value due to a one-year CMC-related delay.
What role do stability studies play in CMC?
Stability studies, designed and analyzed by CMC statisticians, monitor how environmental factors affect a medication's potency and shelf life, ensuring that products remain safe and effective throughout their lifecycle.
How does effective management of CMC elements benefit product development?
Efficient management of CMC elements can prevent unnecessary delays in product development, which is crucial as the complexity of pharmaceutical modalities increases.
What is the overall conclusion regarding the significance of CMC in pharmaceuticals?
The CMC meaning in pharma is a foundational element that guarantees the quality and safety of pharmaceutical products, ultimately protecting patient health and enhancing the credibility of the pharmaceutical industry.




