Overview
In the pharmaceutical sector, the full form of BMR stands for Batch Manufacturing Records. These documents are essential, detailing every aspect of the production process for pharmaceutical products. Their critical role lies in ensuring compliance and product safety.
- Comprehensive documentation, coupled with regular audits, is vital in maintaining high standards.
- Furthermore, the integration of AI technologies significantly enhances traceability, quality assurance, and adherence to regulatory standards in pharmaceutical manufacturing.
- This underscores the necessity of BMRs in fostering a robust and reliable production environment.
Introduction
Understanding the intricacies of Batch Manufacturing Records (BMRs) is essential for anyone involved in the pharmaceutical industry. These comprehensive documents ensure compliance with stringent regulatory standards and play a pivotal role in maintaining product safety and quality.
As manufacturers increasingly integrate advanced technologies like AI into their processes, the potential for enhancing BMR management becomes a focal point of discussion.
How can companies navigate the complexities of BMR compliance while leveraging innovative solutions to enhance operational efficiency and product integrity? This question invites further exploration into the intersection of compliance and technology, paving the way for actionable insights.
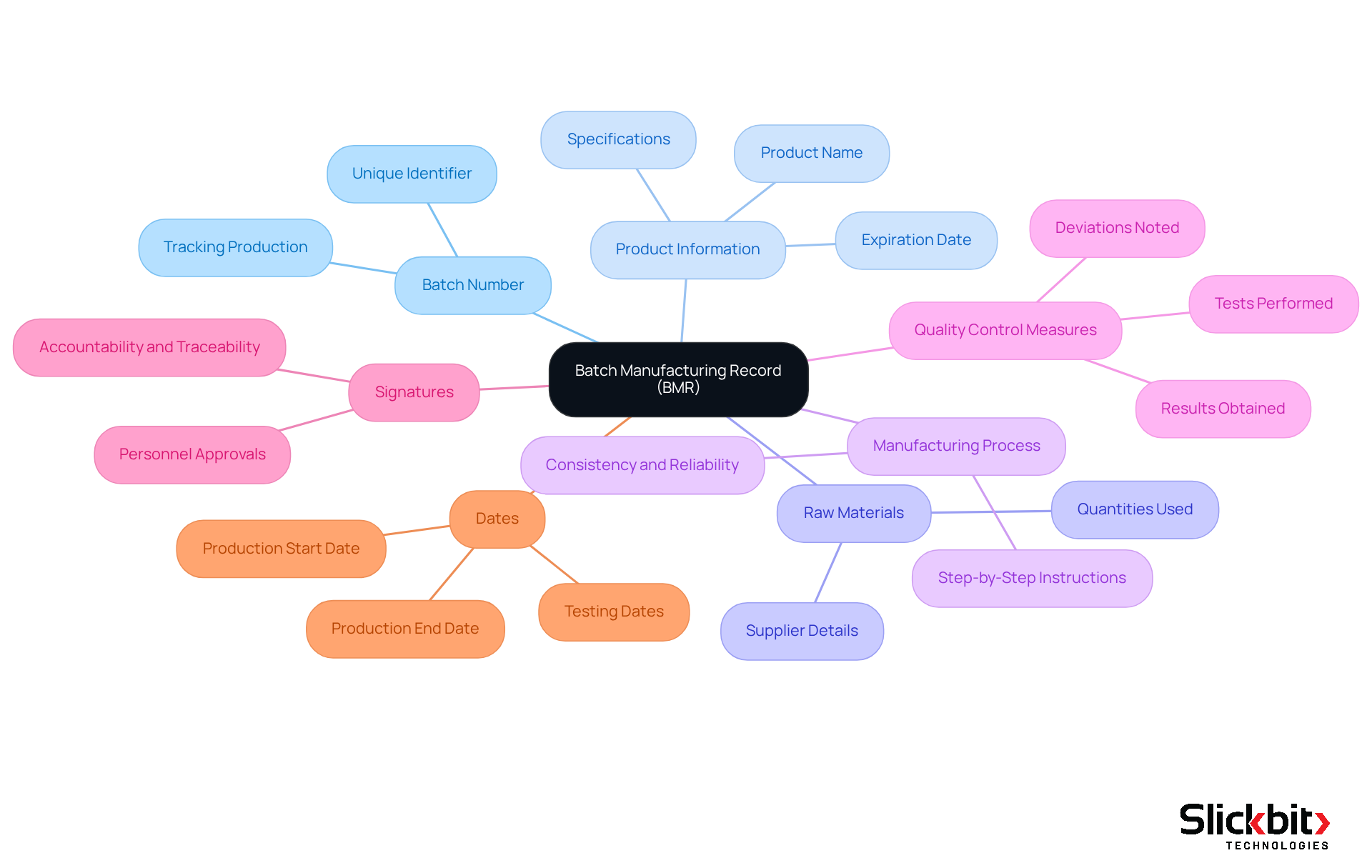
Define Batch Manufacturing Records (BMRs) and Their Importance in Pharma
The term BMR full form in pharma refers to Batch Manufacturing Records, which are comprehensive documents detailing every aspect of the production process for a specific batch of pharmaceutical products. They serve as an essential historical record, encompassing information on:
- Raw materials
- Equipment utilized
- Manufacturing procedures
- Quality control measures
The significance of the BMR full form in pharma is crucial as it ensures that products are manufactured consistently, safely, and in compliance with regulatory standards.
Furthermore, with the advent of AI technologies such as Trend 483, manufacturers can leverage insights from FDA inspections to identify systemic risks and recurring violations. This advancement significantly enhances compliance and quality assurance measures related to BMRs. Trend 483 empowers users to search, filter, and access comprehensive 483s directly, providing deeper insights that can profoundly impact BMR operations.
Consequently, this integration of AI not only improves traceability but also allows manufacturers to monitor the production cycle effectively. By recognizing any deviations from established protocols, they can uphold the highest standards of quality and safety in their operations.
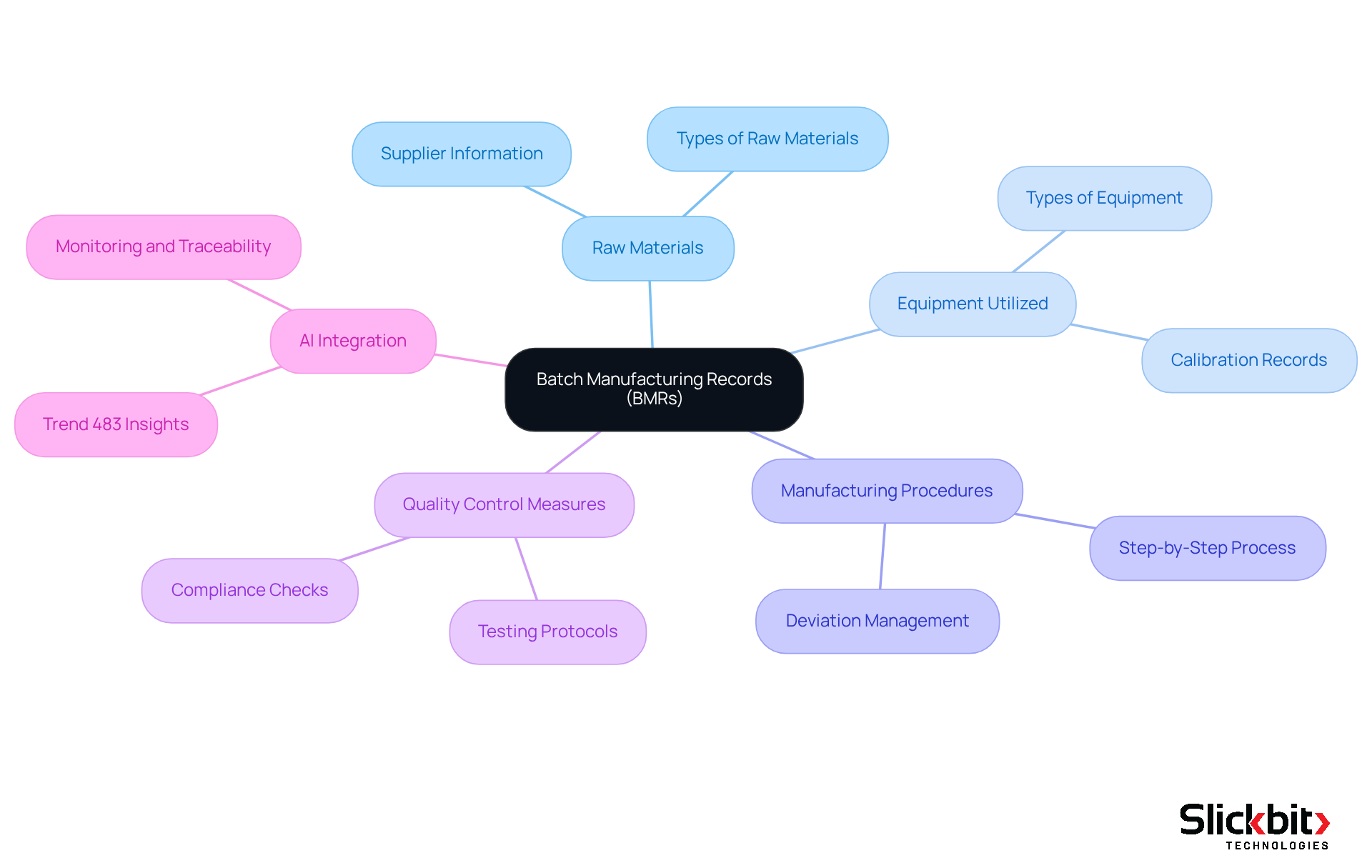
Outline Key Components of a Batch Manufacturing Record
The BMR full form in pharma is a Batch Manufacturing Record, which is a critical document that encompasses several key components essential for ensuring compliance and product safety.
- Batch Number: This unique identifier is crucial for tracking each batch produced.
- Product Information: It includes vital details such as the product name, specifications, and expiration date.
- Raw Materials: Comprehensive information on all materials used is documented, including supplier details and quantities.
- Manufacturing Process: Step-by-step instructions outline the procedures followed during production, ensuring consistency and reliability.
- Quality Control Measures: This section documents tests performed, results obtained, and any deviations noted, reinforcing the commitment to quality.
- Signatures: Required approvals from personnel engaged in the production process ensure accountability and traceability.
- Dates: Significant dates, including production start and end dates, along with any pertinent testing dates, are meticulously recorded.
These components are not merely procedural; they are essential for upholding the standards set by Good Manufacturing Practices (GMP) and understanding the BMR full form in pharma, which ensures product safety. Furthermore, leveraging AI tools such as Slickbit's Trend 483 can significantly enhance the efficiency of overseeing these elements. By recognizing patterns in adherence and identifying systemic risks, these tools ultimately contribute to improved quality assurance in pharmaceutical manufacturing.
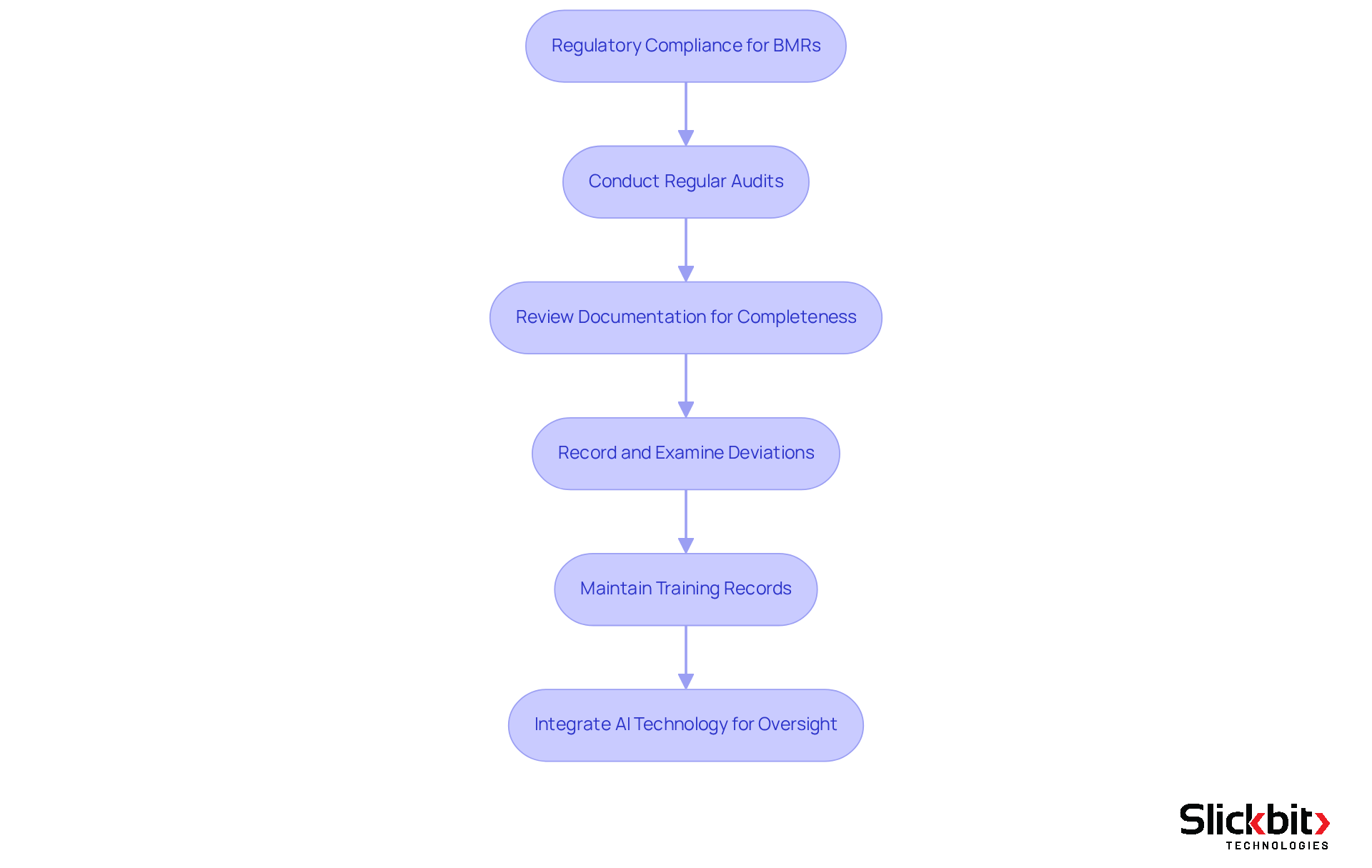
Discuss Regulatory Compliance and Auditing Requirements for BMRs
Regulatory adherence for Batch Manufacturing Records (BMRs), which is the BMR full form in pharma, is governed by various standards, including those set by the FDA and EMA. These regulations mandate that BMRs be complete, accurate, and readily available for inspection. Key auditing requirements are critical for ensuring compliance with the BMR full form in pharma and other regulatory standards.
Regular audits are essential; manufacturers must conduct periodic evaluations of their BMR systems. This process ensures that all documentation is reviewed thoroughly to verify that all required information is present and accurate. Furthermore, any deviations from the established production method must be meticulously recorded and examined, with corrective measures implemented as necessary. Training records are also vital, as personnel involved in the manufacturing process must receive adequate training, and documentation of this training should be maintained.
Integrating AI technology into this framework can significantly enhance oversight of these regulatory requirements. By identifying trends in systemic risks and repeated violations, AI offers deeper insights into BMR adherence. Failure to comply with these requirements can result in severe penalties, including product recalls and legal action, underscoring the importance of rigorous adherence to regulatory standards.
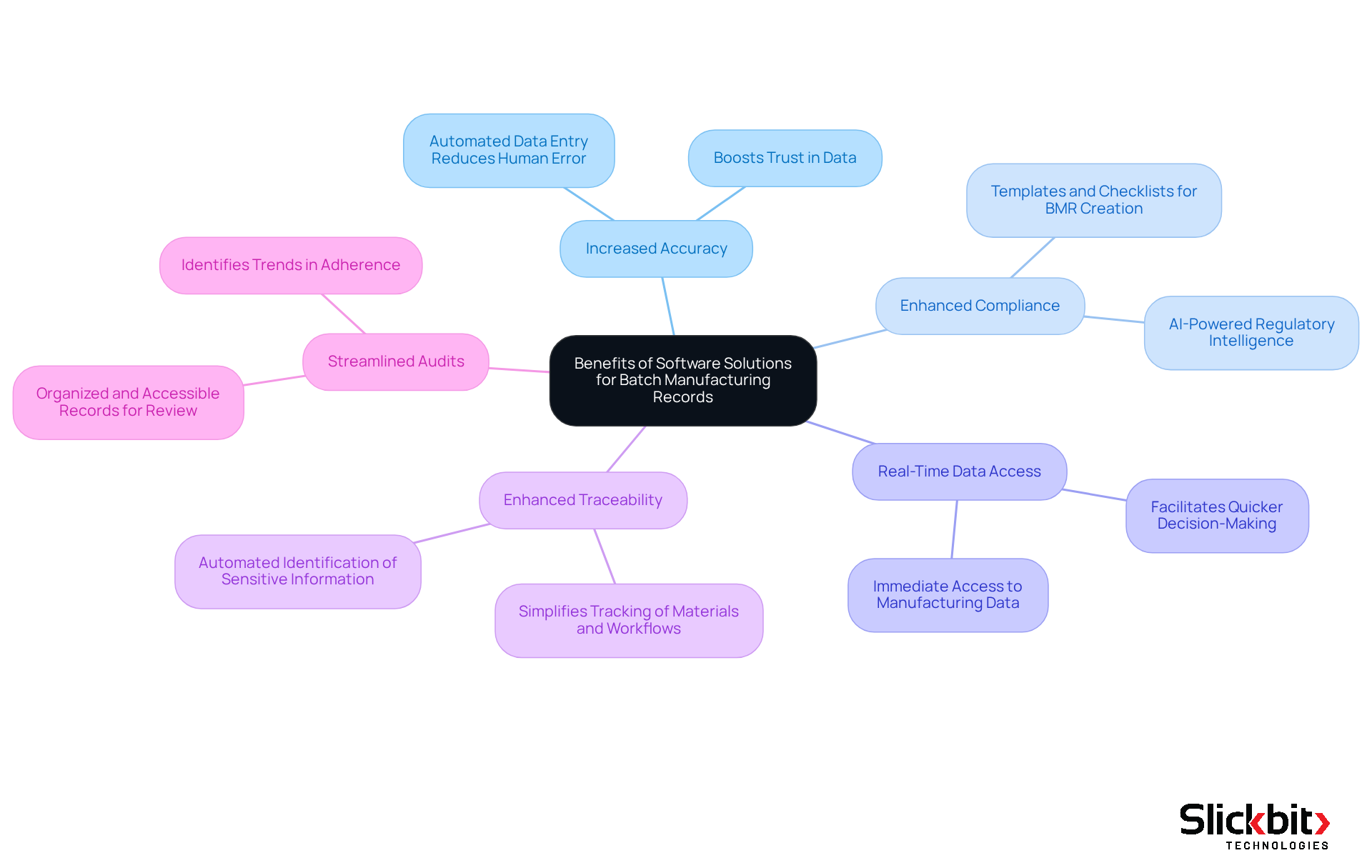
Explore Benefits of Software Solutions for Batch Manufacturing Records
Implementing software solutions for Batch Manufacturing Records, or the BMR full form in pharma, presents significant advantages that can transform operational efficiency.
-
Increased Accuracy: By automating data entry, the risk of human error is substantially diminished, ensuring that records are both precise and reliable. This enhancement not only boosts accuracy but also fosters trust in the data being utilized.
-
Enhanced Compliance: Software plays a crucial role in meeting regulatory requirements by offering templates and checklists for the BMR full form in pharma creation. Notably, Slickbit.ai's AI-powered Regulatory Intelligence, particularly through tools like Trend 483, equips pharmaceutical teams with accurate insights derived from FDA guidance, thereby fortifying compliance efforts.
-
Real-Time Data Access: The transition to digital records enables immediate access to manufacturing data, which facilitates quicker decision-making and problem resolution. This immediacy is essential in a fast-paced industry where timely information can significantly impact outcomes.
-
Enhanced Traceability: Software solutions frequently introduce features that enhance traceability, simplifying the tracking of materials and workflows throughout production. Furthermore, tools like Vault Redact automate the identification and removal of PII and PHI from documents, ensuring that sensitive information is managed securely and responsibly.
-
Streamlined Audits: Digital BMRs simplify the auditing process by providing organized and readily accessible records for review. Insights gained from Trend 483 can also help identify trends in adherence, thereby making audits more efficient and effective.
By leveraging advanced technology, including Slickbit.ai's innovative solutions, pharmaceutical companies can significantly enhance their processes related to the BMR full form in pharma management. This strategic approach ultimately leads to improved compliance and elevated product quality.
Conclusion
The full form of BMR in pharmaceuticals, or Batch Manufacturing Records, serves as a cornerstone of the manufacturing process, ensuring that products are produced safely, consistently, and in compliance with regulatory standards. These comprehensive documents capture intricate production details and play a pivotal role in maintaining quality and accountability throughout the manufacturing cycle.
Key components such as batch numbers, product information, and quality control measures are integral to BMRs, providing a structured approach to tracking and managing pharmaceutical production. Furthermore, the integration of AI technologies, like Trend 483, enhances this process by offering deeper insights into compliance and operational efficiency, thereby reinforcing the importance of meticulous record-keeping in the industry.
Ultimately, the significance of Batch Manufacturing Records extends beyond mere documentation; they are vital for upholding the integrity of pharmaceutical products and ensuring public safety. Embracing software solutions can further streamline BMR management, enabling pharmaceutical companies to enhance accuracy, compliance, and traceability. By prioritizing these practices, the industry can continue to deliver high-quality products that meet the rigorous demands of regulatory standards and consumer safety.
Frequently Asked Questions
What are Batch Manufacturing Records (BMRs) in the pharmaceutical industry?
Batch Manufacturing Records (BMRs) are comprehensive documents that detail every aspect of the production process for a specific batch of pharmaceutical products. They include information on raw materials, equipment used, manufacturing procedures, and quality control measures.
Why are BMRs important in pharmaceuticals?
BMRs are crucial because they ensure that pharmaceutical products are manufactured consistently, safely, and in compliance with regulatory standards. They serve as an essential historical record of the production process.
How do AI technologies like Trend 483 enhance BMR operations?
AI technologies such as Trend 483 help manufacturers leverage insights from FDA inspections to identify systemic risks and recurring violations. This enhances compliance and quality assurance measures related to BMRs, allowing for better monitoring of production cycles.
What benefits does Trend 483 provide to manufacturers?
Trend 483 empowers users to search, filter, and access comprehensive 483s directly, offering deeper insights that can significantly impact BMR operations. This integration improves traceability and helps manufacturers recognize deviations from established protocols.
How do BMRs contribute to quality and safety in pharmaceutical manufacturing?
BMRs contribute to quality and safety by ensuring that all manufacturing processes are documented and adhered to, enabling manufacturers to maintain the highest standards and address any deviations promptly.




