Overview
The article titled "Top 9 Life Science Consulting Jobs for R&D Managers in 2025" identifies essential consulting roles that will be pivotal for R&D managers in the life sciences sector by 2025. It highlights the evolving landscape of life science consulting, emphasizing the integration of advanced technologies and data-driven insights. This evolution aims to enhance operational efficiency and compliance within drug development processes. As the industry adapts, R&D managers must recognize these emerging roles to stay competitive and effective in their strategies.
Introduction
The life sciences sector is experiencing a transformative shift, propelled by rapid technological advancements and an escalating demand for compliance and efficiency. This evolution presents R&D managers with a distinctive opportunity to explore innovative consulting roles that not only enhance operational workflows but also significantly influence drug development outcomes. However, as this landscape continues to evolve, it raises the question: what key challenges and trends will shape life science consulting jobs in 2025? This article examines the top nine consulting opportunities poised to redefine the future for R&D professionals within the life sciences industry.
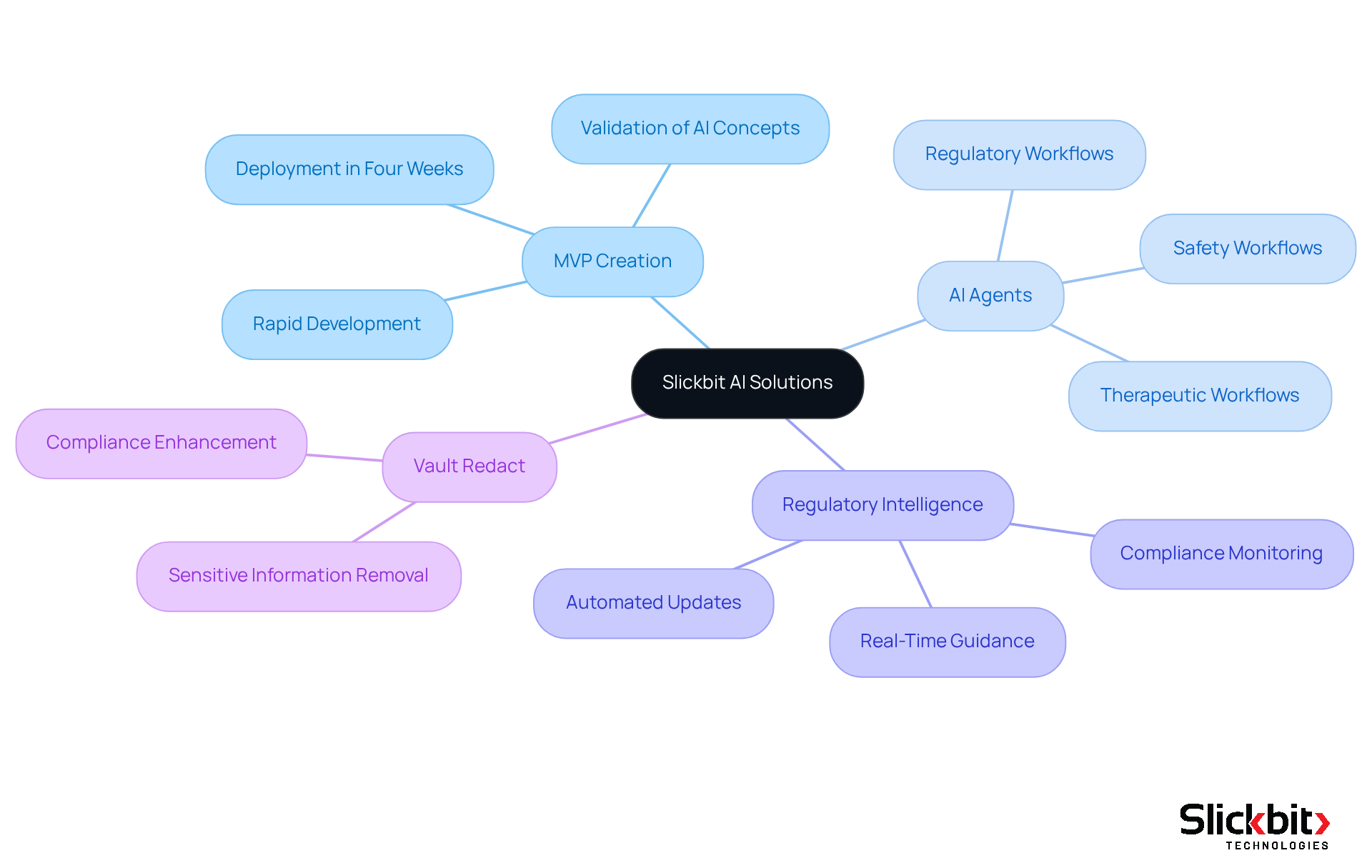
Slickbit: AI Solutions for Life Sciences Compliance and Efficiency
Slickbit.ai stands at the forefront of AI-driven solutions, specializing in enhancing compliance and operational efficiency for life sciences companies. Their rapid MVP creation process empowers organizations to validate AI concepts and deploy functional solutions within a mere four weeks, ensuring adherence to legal frameworks.
With a robust expertise in developing AI agents tailored for regulatory, therapeutic, and safety workflows, coupled with their AI-powered Regulatory Intelligence assistant, Slickbit positions itself as an indispensable partner for R&D managers. This collaboration aims to streamline processes and elevate productivity in drug development and commercialization.
Furthermore, innovative solutions like Vault Redact automate the identification and removal of sensitive information, significantly bolstering compliance efforts within the pharmaceutical industry.
IQVIA: Data-Driven Insights for Life Sciences Innovation
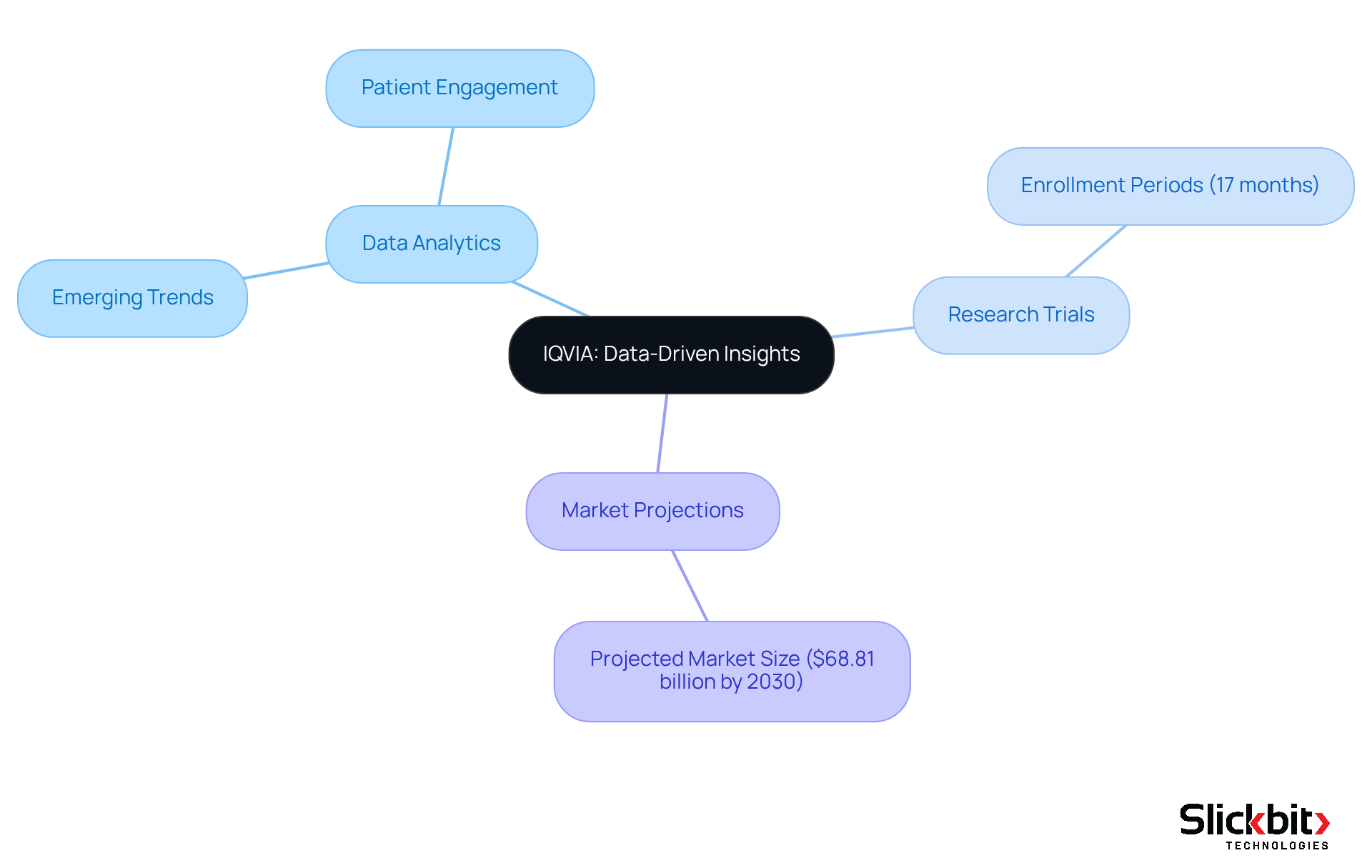
IQVIA stands at the forefront of data analytics in the life sciences sector, offering comprehensive insights that empower organizations to drive innovation. By utilizing extensive datasets, IQVIA enables companies to identify emerging trends, optimize research trials, and enhance patient engagement strategies. This dedication to data-informed decision-making is especially crucial for R&D managers seeking to enhance the efficiency and effectiveness of drug creation processes.
The incorporation of advanced analytics has led to a stabilization of enrollment periods in research trials, which now generally account for 17 months of total time. Furthermore, IQVIA's innovative AI agents have been instrumental in streamlining operations, significantly enhancing the productivity of clinical programs.
As the global life research analytics market is projected to reach $68.81 billion by 2030, the emphasis on data-driven insights will continue to shape the future of drug creation. It is essential for R&D leaders to adopt these strategies to remain competitive.
Bain & Company: Strategic Consulting for Operational Excellence in Life Sciences
Bain & Company focuses on life science consulting jobs, specializing in strategic consulting for the life sciences sector with a strong emphasis on operational excellence. Their consultants work closely with organizations to identify inefficiencies and implement best practices that significantly enhance productivity.
By leveraging advanced analytics and tailored operational strategies, Bain empowers R&D managers to optimize workflows and achieve superior outcomes in drug development. Notably, the integration of innovative methodologies has proven to boost clinical progress productivity, with the composite success rate for drug pipelines rising to 10.8% in 2023, marking the highest level since 2018.
This demonstrates the tangible benefits of strategic consulting in navigating the complexities of drug development, ultimately leading to faster and more efficient treatment solutions.
Amgen: Biotech Consulting for Enhanced Drug Development
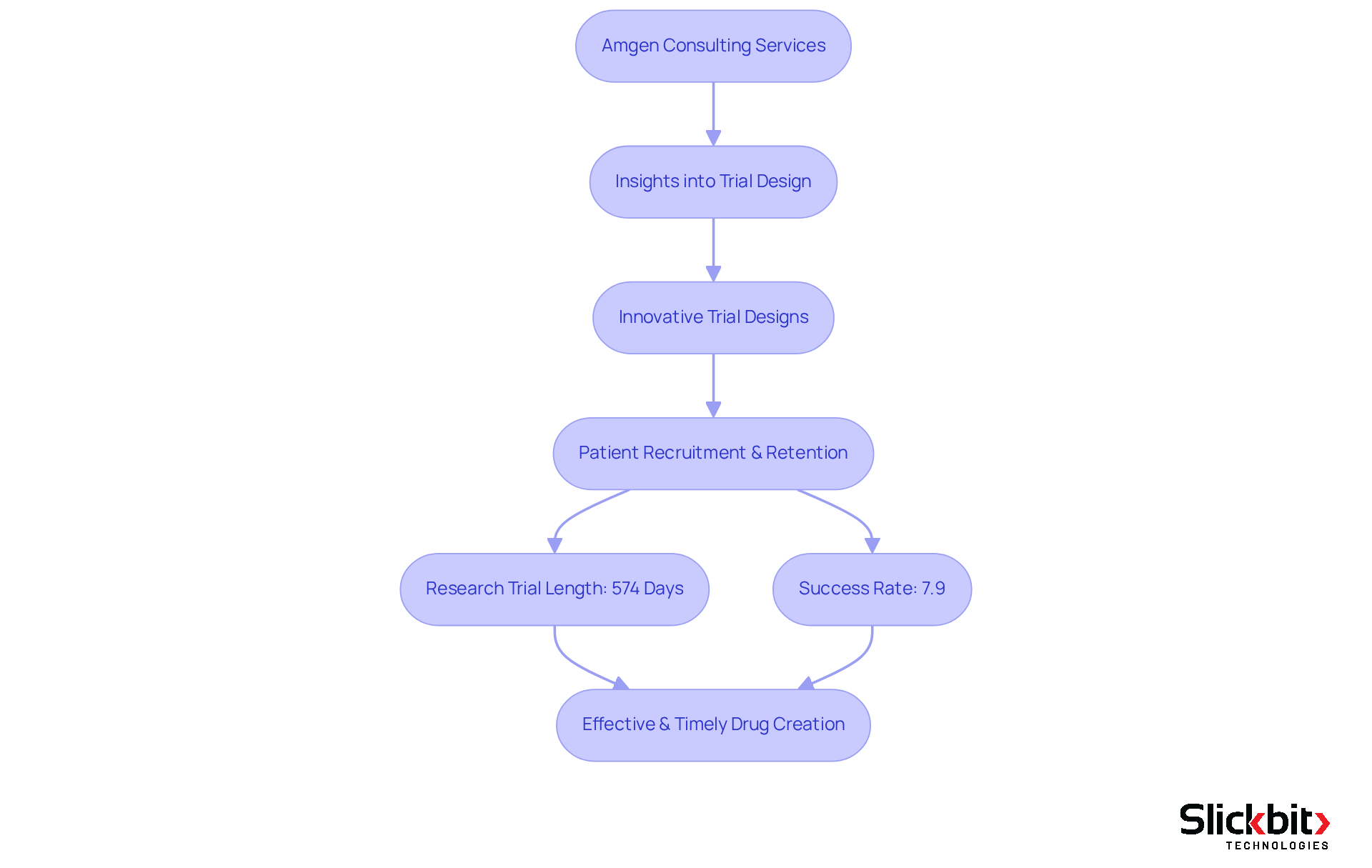
Amgen stands at the forefront of biotechnology, delivering consulting services that significantly enhance drug creation processes. With profound expertise in biopharmaceuticals, Amgen equips R&D managers with essential insights into trial design, regulatory strategies, and market access. Their knowledge is particularly invaluable in navigating the complexities of drug development, ensuring that product launches are not only successful but also compliant with evolving regulations. As Robert A. Bradway, Amgen’s chairman and CEO, stated, "This new center will empower our scientists with the tools and collaborative environment they need to shape the next era of scientific discovery and advance medicines that improve human health."
Recent trends indicate that biotech companies are increasingly focusing on innovative trial designs, which have been shown to enhance patient recruitment and retention rates. For instance, the average length of research trials has been reduced to approximately 574 days, reflecting a concerted effort to streamline processes and boost efficiency. However, it is crucial to recognize that the overall success rate of medical trials remains only 7.9%, highlighting the inherent risks associated with drug innovation.
By leveraging life science consulting jobs from Amgen, R&D managers can implement best practices in clinical trial design, ultimately leading to more effective and timely drug creation outcomes.
Corning Incorporated: Advanced Materials for Life Sciences Research
Corning Incorporated stands at the forefront of advanced materials essential for life sciences research. Their innovative products, including labware and cell culture systems, empower researchers to conduct experiments with greater efficiency and effectiveness. Furthermore, R&D managers can leverage Corning's cutting-edge materials to enhance their research capabilities, thereby driving innovation in drug development. By integrating these advanced solutions, organizations can position themselves to lead in the competitive landscape of pharmaceutical research.
Medidata Solutions: Cloud-Based Clinical Trial Management
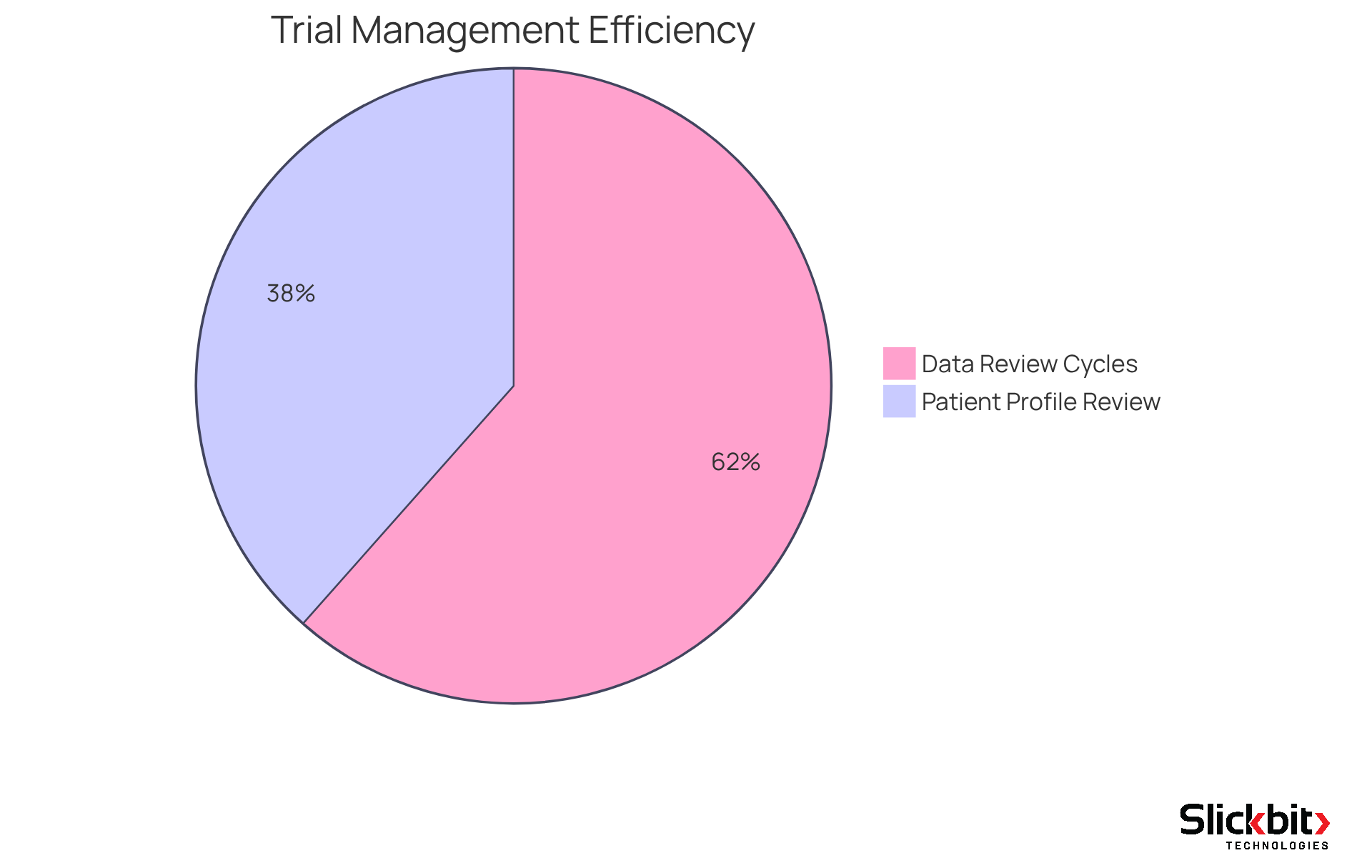
Medidata Solutions presents a robust cloud-based platform designed to enhance trial management. By streamlining data collection and fostering collaboration among stakeholders, their solutions markedly improve trial efficiency.
For example, the Clinical Data Studio has demonstrated the ability to:
- Accelerate data review cycles by up to 80%
- Reduce patient profile review times by 50%
This remarkable efficiency empowers R&D managers to ensure timely and compliant study execution, ultimately leading to the expedited delivery of new therapies to the market.
Furthermore, the platform's integration capabilities provide real-time data access, enabling proactive decision-making and effective risk management throughout the trial lifecycle. As Medidata's Clinical Data Studio tackles these challenges by offering a unified, AI-powered data quality management platform, it underscores the company's pivotal role in transforming clinical trial processes, thereby supporting R&D teams in achieving operational excellence.
Veeva Systems: Cloud Solutions for Regulatory Compliance in Life Sciences
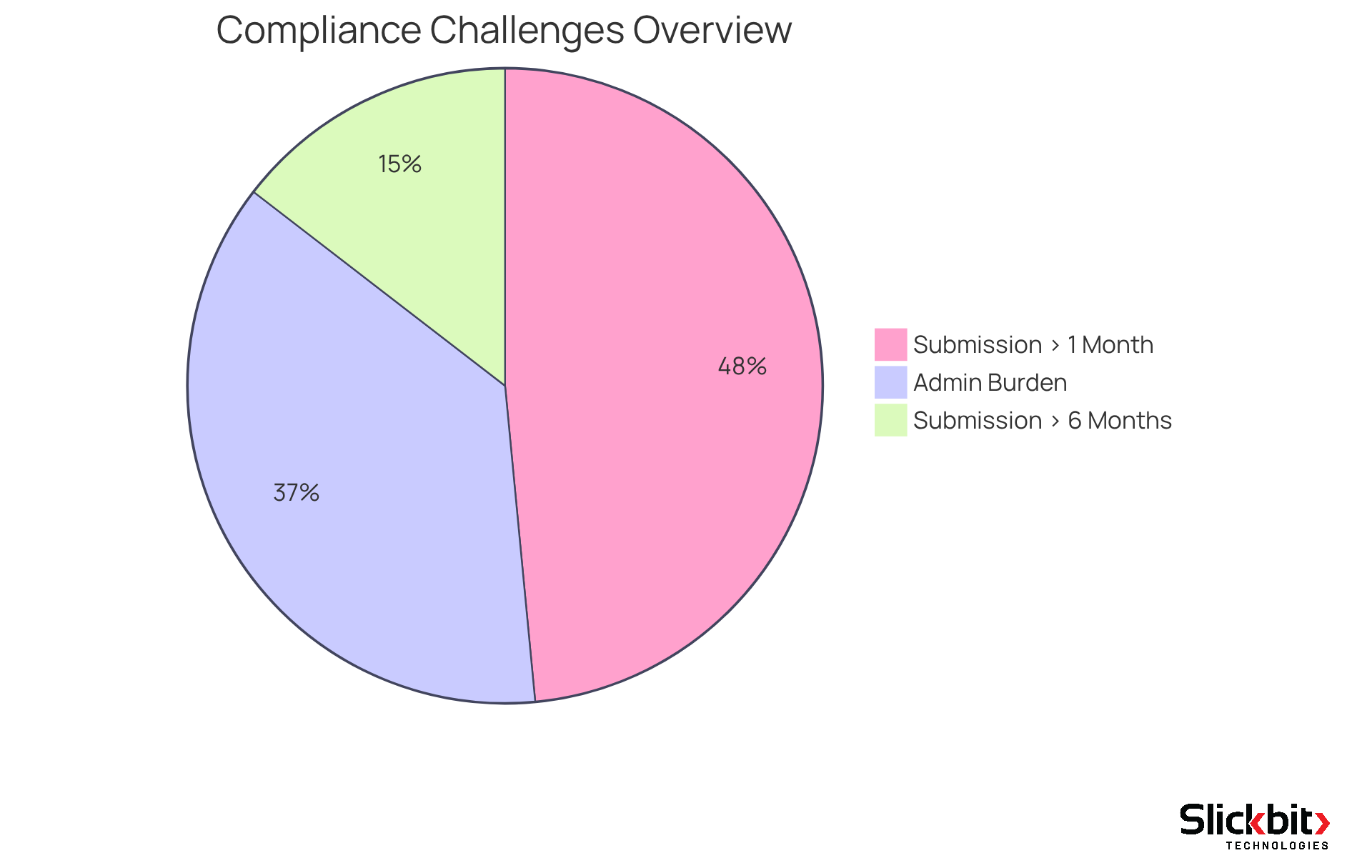
Veeva Systems emerges as a frontrunner in delivering cloud-based software solutions specifically designed for the life sciences industry. Their innovative products significantly enhance compliance by optimizing document management, streamlining submission processes, and improving quality control. Notably, 61% of organizations report facing a considerable administrative burden in compliance matters, highlighting the necessity for effective solutions. Veeva's offerings empower R&D managers to ensure that their operations not only adhere to stringent guidelines but also enhance overall efficiency.
Furthermore, with 80% of companies taking a month or longer to prepare 510(k) submissions, and 24% requiring more than six months, Veeva's cloud solutions can drastically reduce this timeline. This capability facilitates quicker market access for new treatments, a critical factor in the competitive landscape of life sciences. By integrating Veeva's Information Management (RIM) platform, utilized by over 450 companies—including 19 of the largest biopharmaceutical firms—organizations can centralize their compliance data. This addresses the concerns of 50% of respondents who lack confidence in their data quality.
This interconnected approach to document management fosters a culture of quality essential for success in life science consulting jobs within the life sciences sector. To maximize the benefits of Veeva's solutions, R&D managers should prioritize the adoption of RIM systems. This strategic move simplifies compliance processes and enhances data quality, ultimately driving operational excellence.
PAREXEL International: Consulting for Clinical Development and Regulatory Strategy
PAREXEL International specializes in advisory services that prioritize medical advancement and compliance strategy, offering essential support to life-related companies navigating the intricate landscape of clinical trials. Their expertise is crucial in ensuring adherence to evolving legal requirements, a concern that 72% of life sciences executives identify as a top challenge.
R&D managers can leverage PAREXEL's insights to refine their clinical development processes, significantly increasing the likelihood of successful product approvals. Notably, the firm’s consulting group aided nearly one-third of all sponsor NDA/BLA submissions authorized by the FDA in 2024, underscoring their effectiveness in guiding clients through complex compliance frameworks.
Furthermore, with the global pharmaceutical compliance affairs market projected to grow at a CAGR of 7.17% from 2025 to 2030, utilizing such strategic insights is vital for maintaining a competitive edge in the industry. In addition, incorporating AI insights into governance strategies can further bolster compliance efforts, enabling R&D managers to stay ahead of industry challenges.
Consequently, to navigate these challenges effectively, R&D managers should consider engaging with PAREXEL to customize their regulatory strategies, ensuring alignment with market growth and compliance demands.
Deloitte: Integrated Consulting for Life Sciences Transformation
Slickbit provides integrated consulting services that are essential for transforming life science consulting jobs in the life sciences sector. Their comprehensive approach combines strategy, technology, and operations, enabling organizations to adeptly navigate the industry's complexities. R&D managers can leverage Slickbit's extensive expertise to drive innovation, streamline operations, and achieve sustainable growth.
As the life sciences sector evolves, the emphasis on digital advancements and technological innovations becomes increasingly vital. Insights drawn from the restaurant sector's AI transformation reveal crucial lessons for life sciences applications, particularly in the strategic deployment of AI solutions. Executives express optimism about these transformations, with 75% indicating confidence in the industry's future. Furthermore, nearly 60% of leaders plan to increase their investments in generative AI, recognizing its potential to enhance operational efficiency and boost R&D productivity.
Successful transformations, such as advancements in personalized medicine, highlight the significance of integrated consulting in realizing measurable outcomes. By focusing on these strategic initiatives, Slickbit empowers life science consulting jobs to help organizations enhance operational efficiency and adapt to evolving market demands, while also addressing challenges such as pricing and access to medications.
With rapid AI MVP development and comprehensive training available in just four weeks, Slickbit is strategically positioned to support the life sciences sector.
Conclusion
The landscape of life science consulting is rapidly evolving, presenting R&D managers with a wealth of opportunities to enhance their operations and drive innovation. As emphasized in this article, integrating advanced technologies and strategic insights from leading firms such as Slickbit, IQVIA, and Bain & Company is crucial for navigating the complexities of drug development and regulatory compliance. These partnerships not only streamline processes but also cultivate a culture of operational excellence, essential for success in the competitive life sciences sector.
Key insights from the article illustrate that leveraging data-driven analytics, AI solutions, and strategic consulting can significantly enhance productivity and compliance in drug development. Companies like Amgen and Medidata Solutions exemplify the importance of innovative trial designs and cloud-based management systems, which are vital for improving efficiency and reducing time to market. Furthermore, the focus on regulatory strategy and compliance from firms such as PAREXEL and Veeva Systems underscores the necessity for R&D managers to embrace comprehensive solutions that address both operational challenges and market demands.
Ultimately, the future of life science consulting in 2025 presents a call to action for R&D managers. By embracing these emerging trends and technologies, they can position their organizations for success, ensuring they not only keep pace with industry advancements but also lead in delivering innovative therapies that improve patient outcomes. The insights shared in this article serve as a roadmap for navigating the complexities of the life sciences landscape, empowering professionals to make informed decisions that drive sustainable growth and operational excellence.
Frequently Asked Questions
What is Slickbit.ai and what services do they provide?
Slickbit.ai specializes in AI-driven solutions aimed at enhancing compliance and operational efficiency for life sciences companies. They focus on rapid MVP creation to validate AI concepts and deploy functional solutions within four weeks.
How does Slickbit.ai support drug development and commercialization?
Slickbit.ai develops AI agents tailored for regulatory, therapeutic, and safety workflows, and offers an AI-powered Regulatory Intelligence assistant to streamline processes and elevate productivity for R&D managers.
What innovative solution does Slickbit.ai offer for compliance in the pharmaceutical industry?
Slickbit.ai offers Vault Redact, which automates the identification and removal of sensitive information, significantly improving compliance efforts within the pharmaceutical industry.
What role does IQVIA play in the life sciences sector?
IQVIA provides data-driven insights that empower organizations to drive innovation in life sciences through extensive datasets, enabling the identification of emerging trends and optimization of research trials.
How does IQVIA enhance the efficiency of drug creation processes?
IQVIA utilizes advanced analytics to stabilize enrollment periods in research trials and has developed innovative AI agents to streamline operations, significantly boosting the productivity of clinical programs.
What is the projected growth of the global life research analytics market?
The global life research analytics market is projected to reach $68.81 billion by 2030, emphasizing the importance of data-driven insights for R&D leaders to remain competitive.
What services does Bain & Company offer to the life sciences sector?
Bain & Company specializes in strategic consulting focused on operational excellence, helping organizations identify inefficiencies and implement best practices to enhance productivity in the life sciences sector.
How has Bain & Company impacted drug development productivity?
Bain's integration of advanced analytics and tailored operational strategies has led to a rise in the composite success rate for drug pipelines to 10.8% in 2023, the highest level since 2018, demonstrating the benefits of strategic consulting in drug development.




