Overview
This article delves into the significance and implications of Overall Equipment Effectiveness (OEE) within the realm of pharmaceutical R&D. Understanding OEE is crucial for R&D managers, as it serves as a key indicator for optimizing productivity and ensuring compliance. By grasping the nuances of OEE, managers can pinpoint inefficiencies that hinder operational performance.
Furthermore, implementing tailored strategies based on OEE insights can lead to substantial enhancements in drug development timelines and cost efficiency.
In conclusion, a thorough understanding of OEE is not merely advantageous; it is essential for driving forward the success of pharmaceutical R&D initiatives.
Introduction
Understanding Overall Equipment Effectiveness (OEE) is essential for pharmaceutical R&D managers who aim to enhance productivity and streamline operations. By concentrating on OEE, organizations can identify inefficiencies, drive substantial improvements, and ultimately expedite drug development processes. However, as the industry evolves, a pressing challenge persists: how can R&D leaders effectively implement OEE strategies while navigating the complexities of compliance and innovation? This article explores ten key insights designed to empower managers to optimize OEE and transform their operational landscape.
Slickbit: AI Solutions for Optimizing OEE in Pharmaceutical R&D
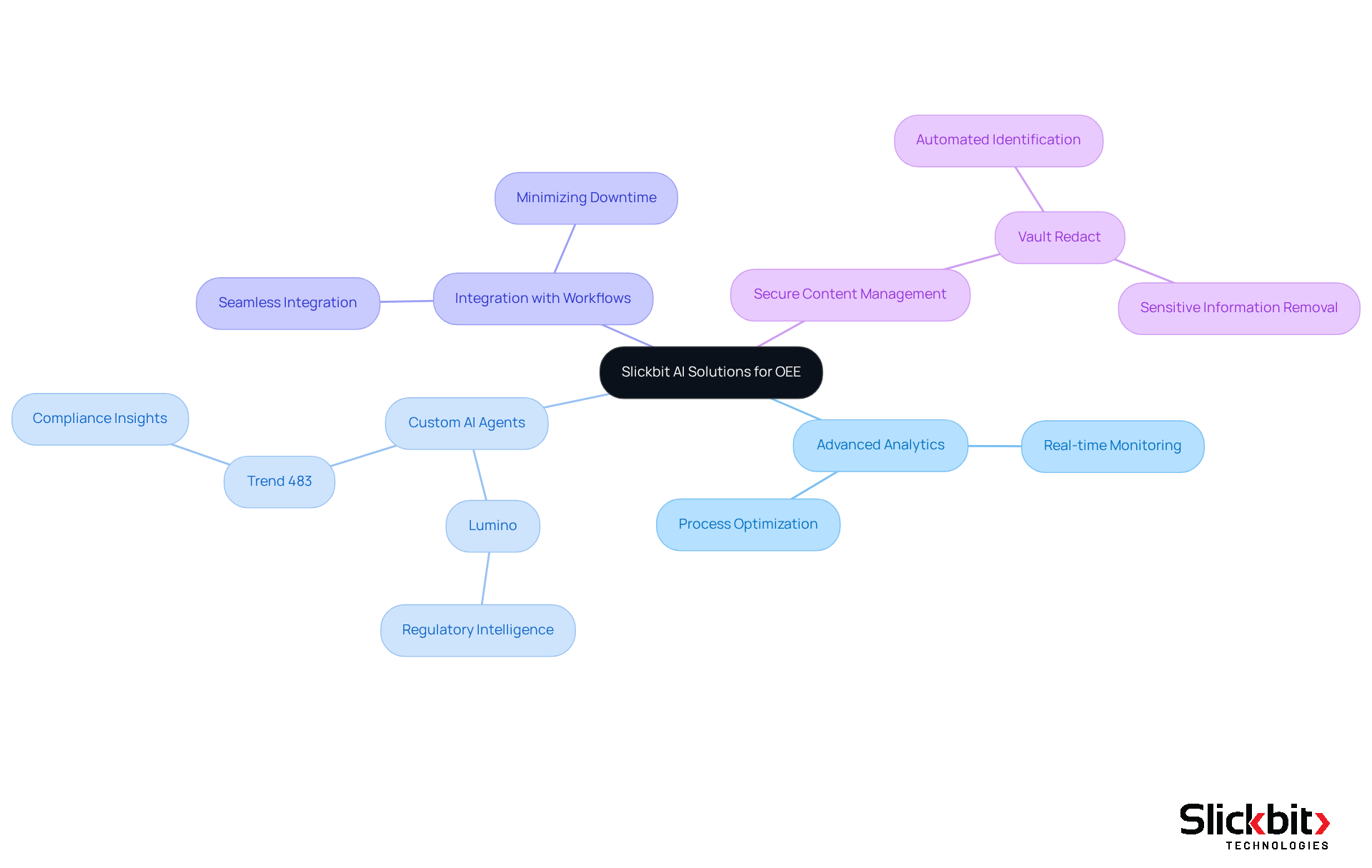
Slickbit.ai stands at the forefront of AI solutions that enhance Overall Equipment Effectiveness (OEE), which reflects the OEE meaning, within research and development for the drug industry. By harnessing advanced analytics and custom AI agents—such as Lumino for regulatory intelligence and Trend 483 for compliance insights—Slickbit empowers R&D managers to streamline processes, minimize downtime, and uphold regulatory standards.
The seamless integration of AI into existing workflows facilitates real-time monitoring and optimization of equipment performance, ultimately propelling productivity and innovation in drug development. Furthermore, solutions like Vault Redact ensure secure content management by automating the identification and removal of sensitive information, thereby bolstering compliance efforts within the industry.
Overall Equipment Effectiveness (OEE): Definition and Importance
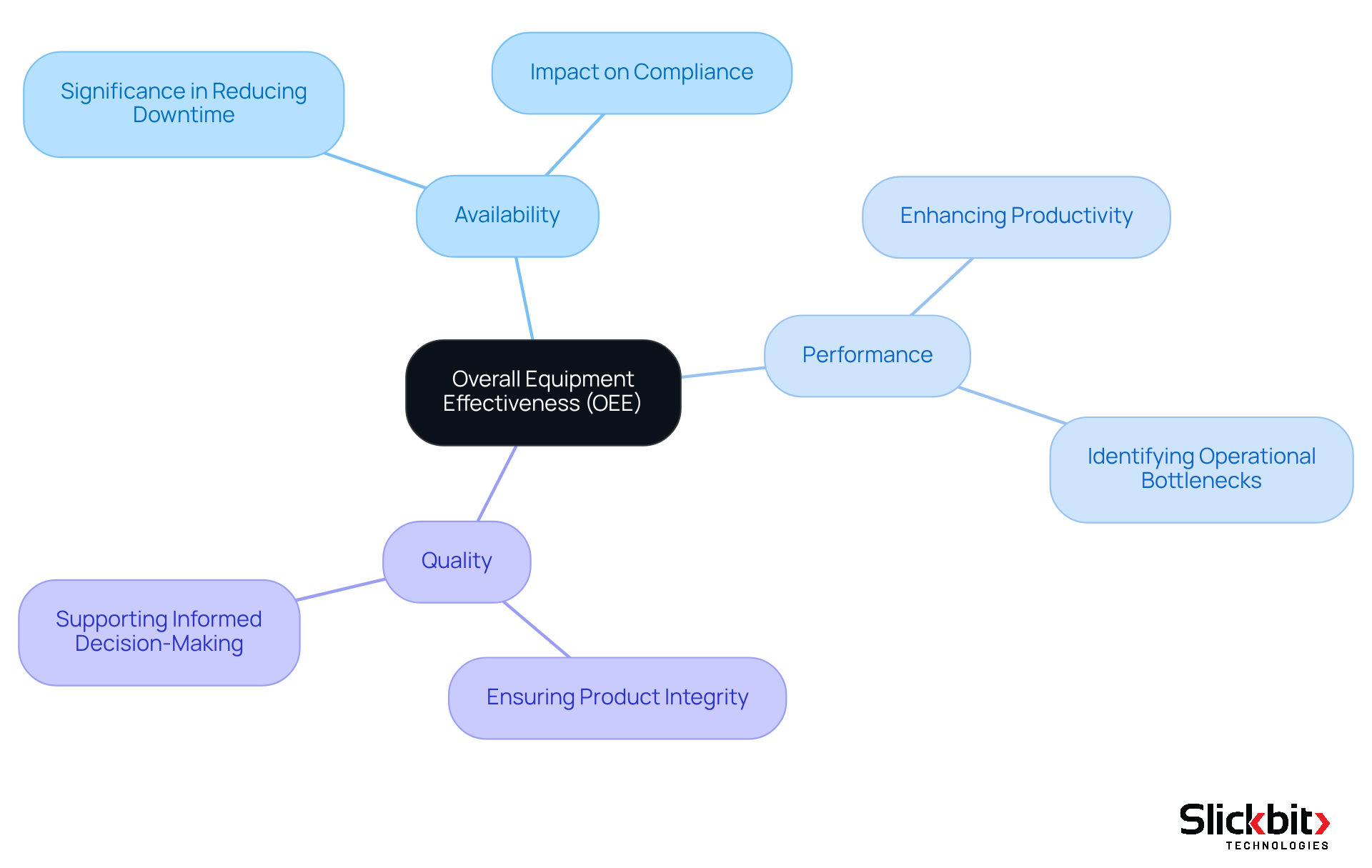
The OEE meaning, or Overall Equipment Effectiveness, is a crucial performance indicator in drug manufacturing, encompassing three fundamental components: availability, performance, and quality. In the context of pharmaceutical R&D, the OEE meaning is vital for pinpointing inefficiencies, reducing waste, and enhancing productivity.
By concentrating on the OEE meaning, R&D leaders can uncover operational bottlenecks and implement targeted improvements that not only enhance compliance but also maximize output. For example, organizations that have embraced OEE metrics report a 10 to 30 percent improvement in their understanding of key insights and trends, facilitating more informed decision-making.
Furthermore, case studies illustrate that companies effectively leveraging OEE can achieve substantial gains in operational efficiency, ultimately resulting in accelerated drug development timelines and decreased costs. As the healthcare landscape evolves, prioritizing OEE will be essential for R&D leaders aiming to drive innovation while ensuring adherence to regulatory standards.
OEE Calculation: Key Factors and Formula for Accurate Measurement
The OEE meaning, or Overall Equipment Effectiveness, is a critical metric calculated using the formula: OEE = Availability × Performance × Quality. For R&D managers aiming to optimize production processes, understanding the OEE meaning is essential. Each component of OEE is defined as follows:
- Availability is the ratio of actual operating time to planned production time.
- Performance is the ratio of actual output to the maximum possible output during the operating time.
- Quality is the ratio of good units produced to the total units produced.
By comprehensively understanding these factors, R&D managers can accurately assess their OEE, identify specific areas for improvement, and implement strategies that enhance operational efficiency.
Benefits of High OEE Scores: Driving Efficiency in Pharmaceutical Development
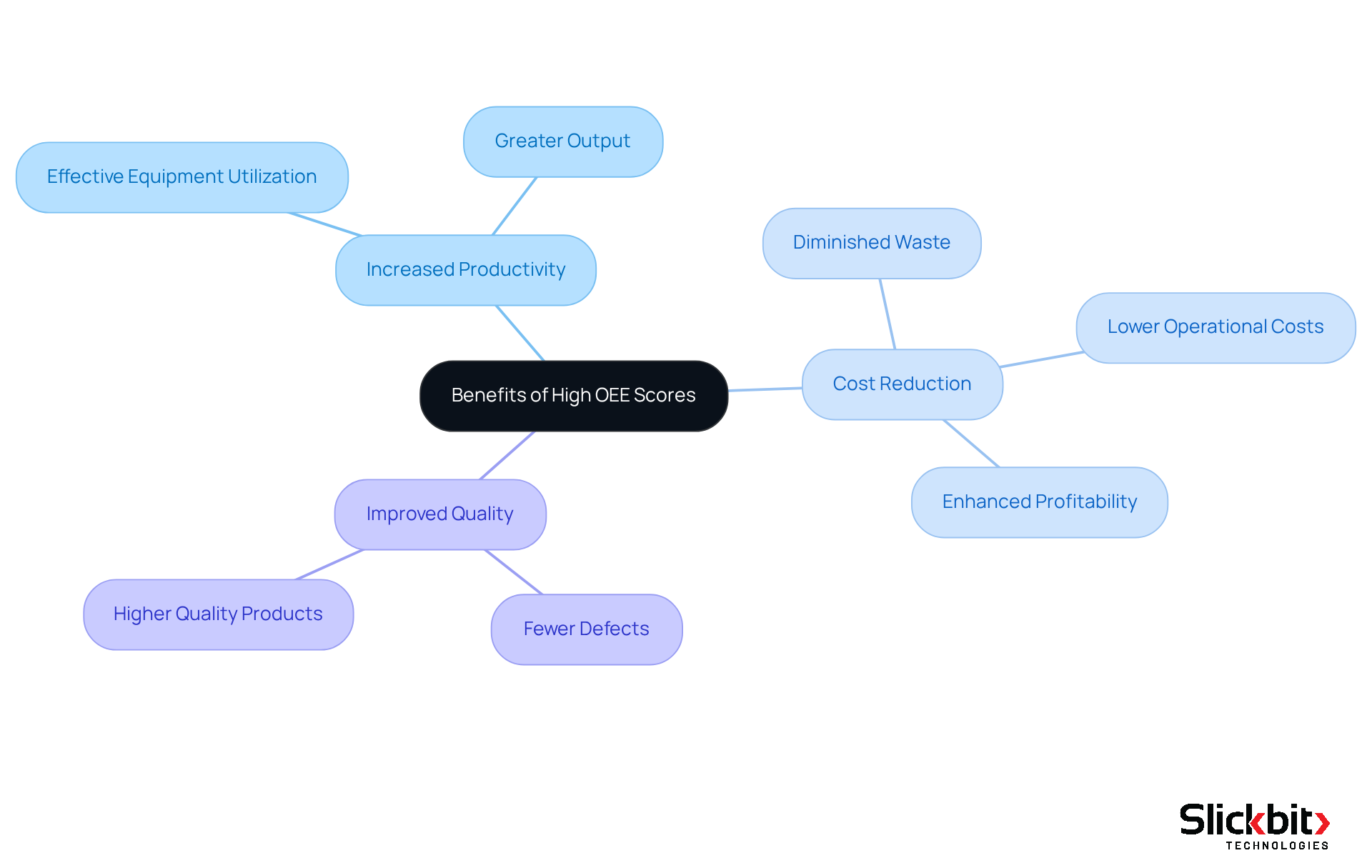
Understanding OEE meaning is crucial as achieving high OEE scores presents significant advantages for pharmaceutical development.
- Increased Productivity is a primary benefit; higher OEE signifies that equipment is utilized more effectively, resulting in greater output.
- Cost Reduction follows closely, as optimizing processes diminishes waste and operational costs, ultimately enhancing overall profitability.
- Additionally, Improved Quality is essential in the medicine sector; high OEE often correlates with fewer defects and the production of higher-quality products.
By concentrating on the OEE meaning, R&D leaders can drive substantial enhancements in their development processes, leading to a more efficient and effective pharmaceutical landscape.
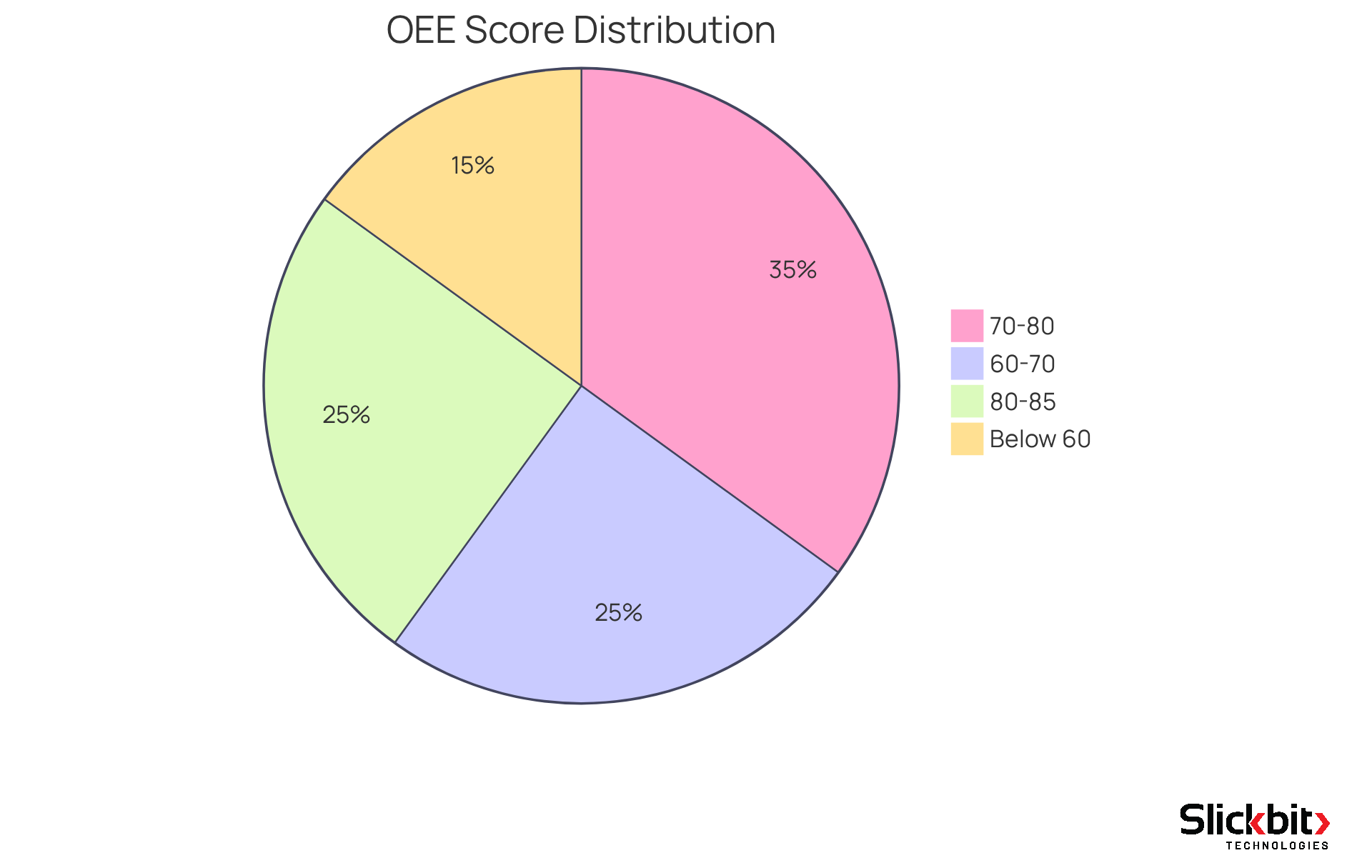
OEE Benchmarks: Setting Performance Standards in Pharma R&D
The OEE meaning varies across industries; however, in pharmaceutical R&D, a typical OEE score falls between 60% and 85%. Establishing performance standards grounded in these benchmarks empowers R&D managers to assess their operations against recognized industry norms. Furthermore, consistently evaluating OEE scores helps in understanding OEE meaning in relation to these benchmarks, aiding in identifying trends, highlighting areas that require attention, and fostering continuous improvement initiatives. By focusing on these critical metrics, organizations can enhance their operational efficiency and drive innovation.
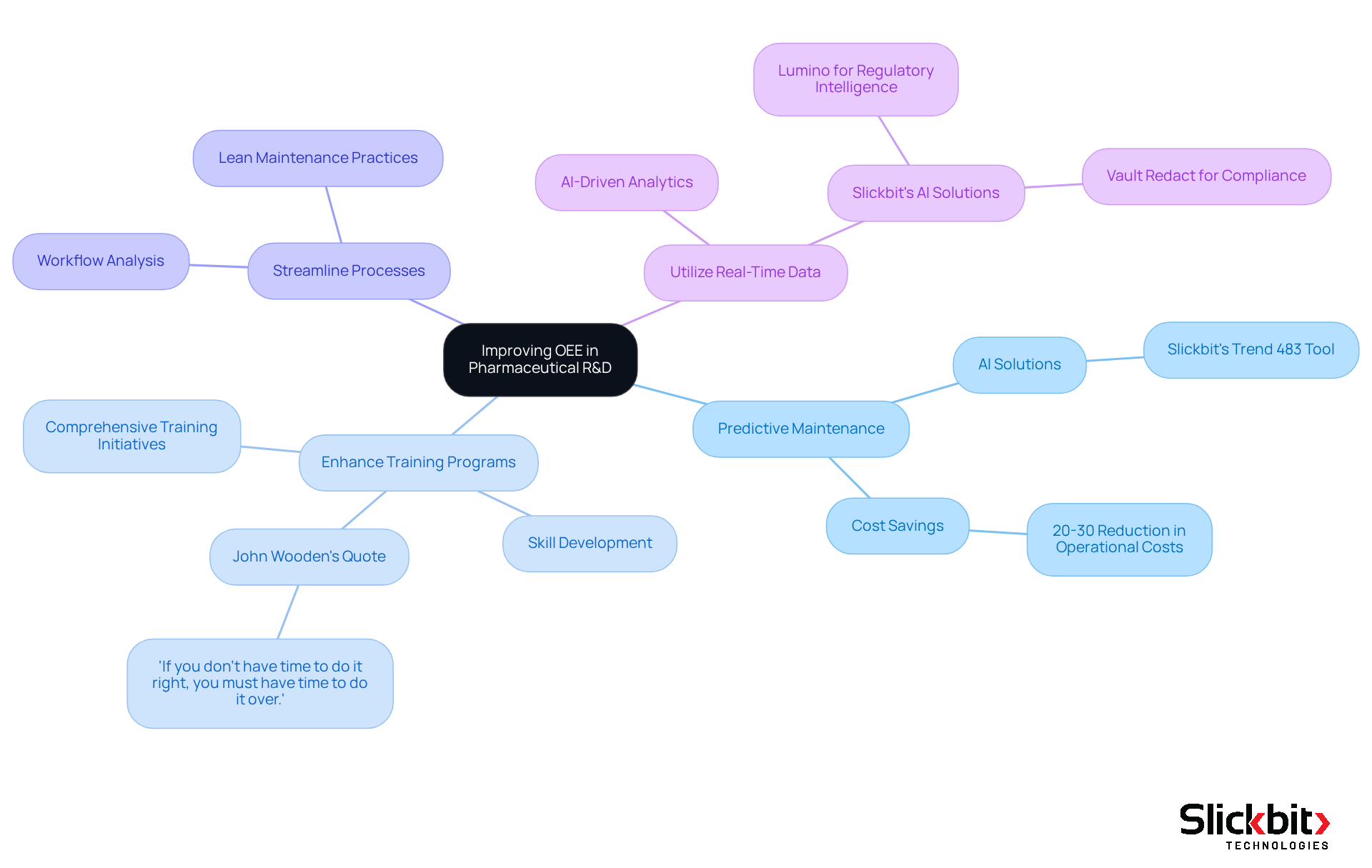
Strategies for Improving OEE: Practical Approaches for R&D Managers
To enhance Overall Equipment Effectiveness (OEE) in pharmaceutical R&D, understanding the OEE meaning is crucial for implementing targeted strategies that address operational challenges head-on.
-
Implement Predictive Maintenance: By leveraging AI solutions, such as Slickbit's Trend 483 tool, organizations can anticipate equipment failures, thereby reducing unplanned downtime and ensuring smoother operations. Notably, generative AI can lead to a 20 to 30 percent savings in operational costs, significantly impacting your bottom line.
-
Enhance Training Programs: Investing in comprehensive training initiatives for staff is essential. Equipping employees with the necessary skills to operate equipment efficiently is paramount. As John Wooden wisely stated, "If you don't have time to do it right, you must have time to do it over," underscoring the importance of thorough training.
-
Streamline Processes: Conducting thorough analyses of existing workflows allows organizations to pinpoint bottlenecks and eliminate redundant steps, fostering a more efficient operational environment. Lean maintenance practices can be particularly beneficial, as they focus on reducing waste and improving productivity.
-
Utilize Real-Time Data: Harnessing AI-driven analytics to continuously monitor equipment performance enables immediate adjustments that can optimize productivity. Slickbit's AI solutions, including Lumino for regulatory intelligence and Vault Redact for compliance, provide insights that further enhance resource management capabilities.
These tactics not only lead to considerable advancements in what is referred to as OEE meaning but also significantly boost overall operational effectiveness within the drug industry.
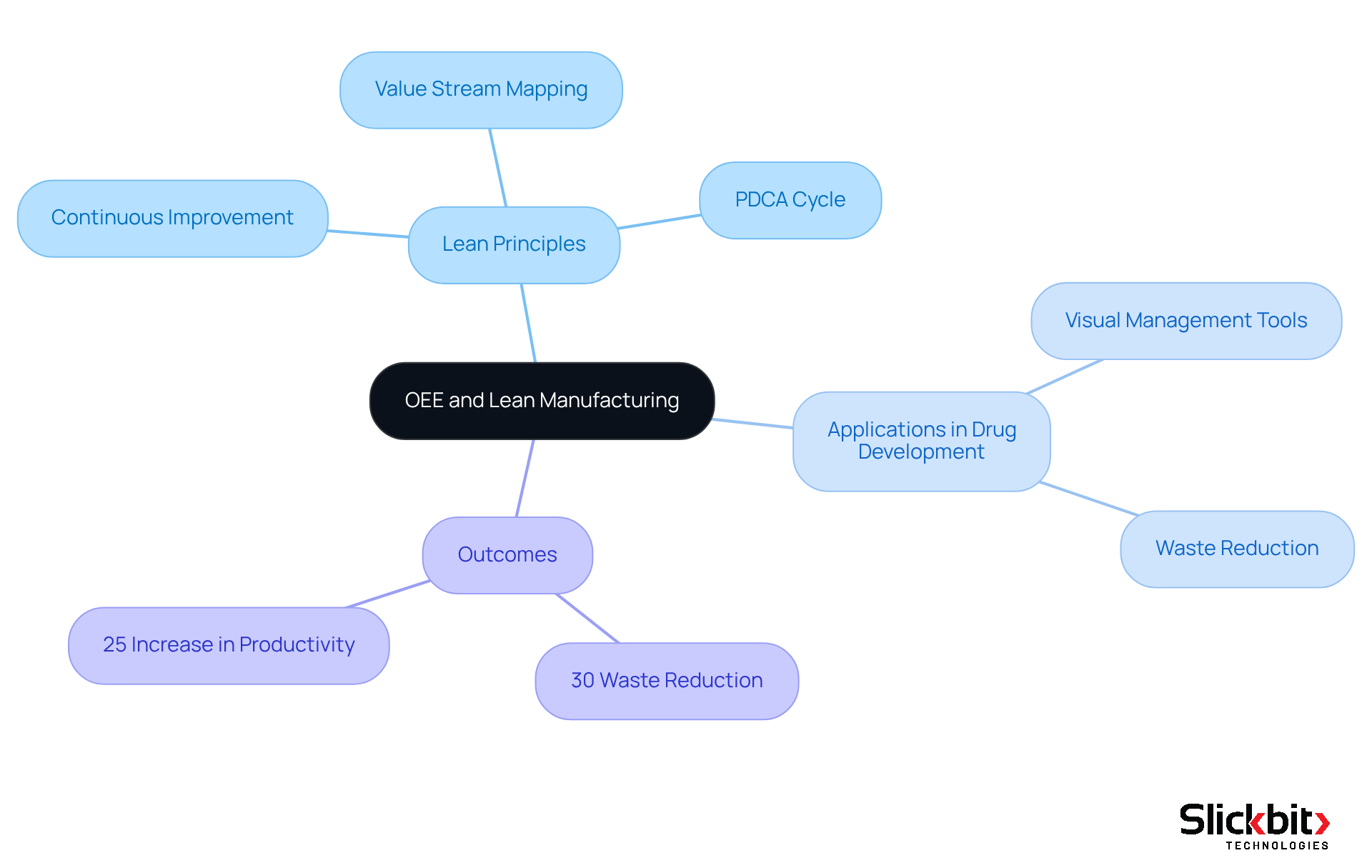
OEE and Lean Manufacturing: Enhancing Efficiency in Drug Development
The concept of Overall Equipment Effectiveness, or OEE meaning, is intrinsically linked to lean manufacturing, as both aim to maximize efficiency while minimizing waste. By implementing lean principles such as continuous improvement and value stream mapping, R&D managers can significantly enhance their understanding of OEE meaning and improve their scores. Lean methodologies empower teams to pinpoint non-value-added activities, streamline processes, and foster a culture of ongoing enhancement. For instance, drug manufacturers that have adopted lean practices report a decrease in waste by as much as 30%, resulting in quicker drug development cycles and improved resource utilization.
Effective applications of lean in the drug industry include:
- The use of visual management tools to monitor workflow and identify bottlenecks, which has led to a 25% increase in productivity in certain cases.
- The application of the PDCA (Plan-Do-Check-Act) cycle, which has proven effective in driving continuous improvement, enabling teams to systematically address inefficiencies.
Lean manufacturing specialists underscore the importance of maximizing efficiency in pharmaceutical R&D. As Taiichi Ohno noted, "The Toyota style is not to create results by working hard; it is a system that says there is no limit to people’s creativity." This mindset encourages R&D teams to innovate and optimize their processes, ultimately leading to enhanced OEE meaning and more effective drug development outcomes. Furthermore, as Shigeo Shingo pointed out, recognizing and eliminating waste is crucial for process optimization. R&D supervisors should regularly evaluate their workflows to identify areas of waste and apply lean practices to improve overall efficiency. By nurturing a culture of ongoing enhancement and actively involving teams in these initiatives, organizations can achieve substantial progress in their drug development processes.
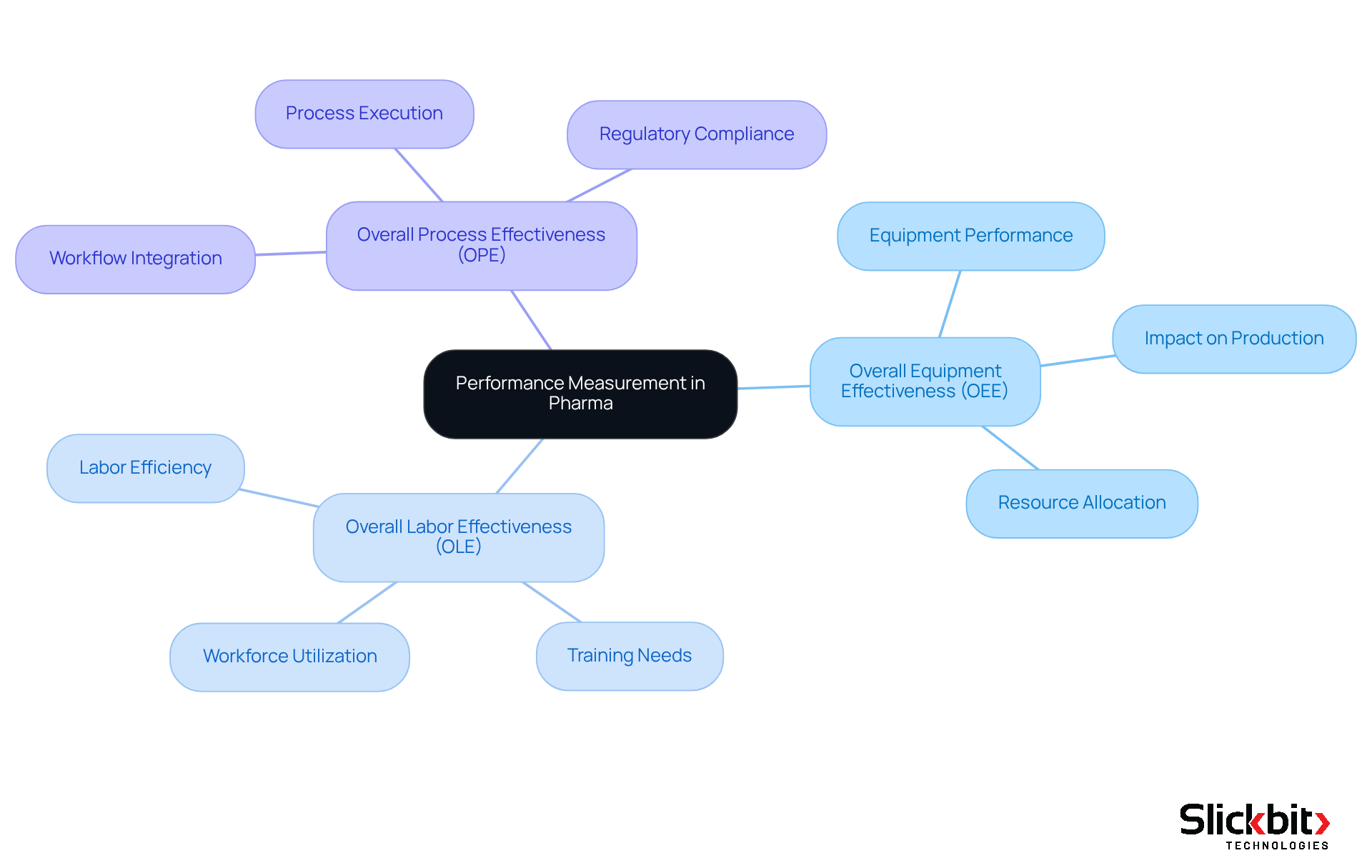
OEE vs. Other Metrics: Understanding Performance Measurement in Pharma
Understanding the OEE meaning and the distinctions between Overall Equipment Effectiveness (OEE), Overall Labor Effectiveness (OLE), and Overall Process Effectiveness (OPE) is crucial for R&D supervisors within the drug industry. The OEE meaning serves as a critical metric that emphasizes equipment performance, while OLE assesses labor efficiency, and OPE sheds light on the effectiveness of the entire process. This holistic approach empowers R&D managers to identify precise areas for enhancement.
For example, OLE can illuminate how efficiently the workforce is utilized, particularly significant in light of studies indicating that employees often spend nearly 50% of their time on activities that do not align with their work objectives. By concurrently measuring OLE and understanding OEE meaning, organizations can identify inefficiencies in labor allocation and training, fostering improved productivity.
In a similar vein, OPE encompasses the entire workflow, providing a comprehensive view of how well processes are integrated and executed. This metric is indispensable in an era where drug companies confront mounting pressures from regulatory demands and the necessity for accelerated development cycles. Indeed, pharmaceutical companies now allocate significantly more resources to R&D while generating markedly fewer new molecules compared to a decade ago.
Industry analysts assert that a robust performance measurement system, which encompasses OEE meaning, OLE, and OPE, is foundational for aligning operational goals with organizational objectives. As Sameer Nagarajan articulates, "Performance management continues to be an important cornerstone of business and individual growth and development." By leveraging these metrics, R&D teams can cultivate a culture of continuous improvement, ultimately yielding superior outcomes in drug development and commercialization.
Case Studies: Successful OEE Implementation in Pharmaceutical R&D
Numerous drug manufacturers have effectively harnessed OEE optimization strategies, which demonstrate the OEE meaning, resulting in significant enhancements in operational efficiency. For instance, a prominent biotech firm utilized AI-driven predictive maintenance, achieving a remarkable 30% reduction in equipment downtime and a subsequent 15% increase in OEE.
In addition, the integration of machine learning algorithms and Databricks in drug manufacturing led to a 30% reduction in task completion time and a 20% improvement in adherence to regulations, further illustrating the impact of technology on OEE enhancements.
Furthermore, Slickbit's Trend 483 tool exemplifies how AI can identify trends in systemic risks and compliance issues, providing deeper insights that enhance operational efficiency.
In another instance, a drug manufacturer adopted lean methodologies to streamline production processes, resulting in a 20% reduction in waste, which correspondingly boosted their OEE.
These examples underscore the transformative potential of OEE optimization, which reflects the OEE meaning in enhancing productivity and efficiency within pharmaceutical R&D. Effective OEE execution also emphasizes the importance of training the team and establishing metrics to ensure ongoing advancement.
FAQs About OEE: Clarifying Common Misconceptions for R&D Managers
In addressing common inquiries regarding Overall Equipment Effectiveness (OEE), understanding the OEE meaning is crucial to provide clarity and insight.
-
Is a high OEE score always good? Not necessarily; it is essential to consider the context and specific operational goals when evaluating OEE scores. A high score may not reflect optimal performance if it does not align with strategic objectives.
-
Can OEE be improved overnight? OEE improvement is a continuous process that demands time and sustained effort. Immediate changes may yield temporary gains, but lasting improvement requires a systematic approach.
-
Is OEE applicable only to manufacturing? While OEE is predominantly utilized in manufacturing settings, its principles extend beyond this realm. In fact, the concepts of OEE can be effectively applied to various areas within pharmaceutical R&D, enhancing overall operational efficiency.
By addressing these frequently asked questions, R&D managers can cultivate a deeper understanding of the OEE meaning and its significant implications for optimizing their operations.
Conclusion
Understanding the meaning of Overall Equipment Effectiveness (OEE) is essential for pharmaceutical R&D managers who aim to enhance operational efficiency and drive innovation in drug development. OEE serves as a pivotal metric that encompasses availability, performance, and quality, enabling organizations to identify inefficiencies and implement targeted strategies for improvement. By prioritizing OEE, R&D leaders can streamline processes while ensuring compliance with regulatory standards, ultimately achieving substantial gains in productivity and cost-effectiveness.
Key insights into OEE underscore its importance within the pharmaceutical landscape:
- The integration of AI solutions, such as those offered by Slickbit, plays a crucial role in optimizing OEE by providing real-time monitoring and predictive analytics.
- The adoption of lean manufacturing principles can significantly enhance OEE scores, fostering a culture of continuous improvement.
- Case studies illustrate the tangible benefits of implementing OEE strategies, showcasing increased productivity and reduced waste across various organizations.
In conclusion, embracing the meaning of OEE and its associated metrics is vital for pharmaceutical R&D managers navigating the complexities of drug development effectively. By leveraging advanced technologies and adopting best practices, organizations can foster a more efficient and compliant environment. The journey toward optimizing OEE is not merely a task; it is a strategic imperative that can lead to transformative outcomes in the pharmaceutical industry.
Frequently Asked Questions
What is Slickbit and how does it relate to OEE in pharmaceutical R&D?
Slickbit is a provider of AI solutions designed to enhance Overall Equipment Effectiveness (OEE) in pharmaceutical research and development. It uses advanced analytics and custom AI agents to help R&D managers streamline processes, minimize downtime, and maintain regulatory standards.
What does OEE stand for and why is it important in the drug manufacturing industry?
OEE stands for Overall Equipment Effectiveness. It is a crucial performance indicator in drug manufacturing that helps identify inefficiencies, reduce waste, and enhance productivity. Focusing on OEE allows R&D leaders to uncover operational bottlenecks and implement improvements that maximize output and ensure compliance.
What are the three components of OEE?
The three components of OEE are availability, performance, and quality. Availability measures the ratio of actual operating time to planned production time; performance assesses the ratio of actual output to the maximum possible output during operating time; and quality evaluates the ratio of good units produced to the total units produced.
How is OEE calculated?
OEE is calculated using the formula: OEE = Availability × Performance × Quality. This calculation helps R&D managers assess their overall equipment effectiveness and identify areas for improvement.
What benefits can companies achieve by leveraging OEE metrics?
Companies that effectively leverage OEE metrics can achieve a 10 to 30 percent improvement in understanding key insights and trends, leading to more informed decision-making. Additionally, they can realize substantial gains in operational efficiency, resulting in accelerated drug development timelines and decreased costs.
What role does AI play in optimizing OEE according to the article?
AI plays a significant role in optimizing OEE by enabling real-time monitoring and optimization of equipment performance. Solutions like Lumino for regulatory intelligence and Trend 483 for compliance insights help R&D managers enhance productivity and innovation in drug development.
How does Slickbit ensure secure content management?
Slickbit ensures secure content management through solutions like Vault Redact, which automates the identification and removal of sensitive information, thereby enhancing compliance efforts within the pharmaceutical industry.




