Overview
This article delves into the crucial elements and best practices for mastering the Pharmacovigilance System Master File (PSMF), a key factor in ensuring regulatory compliance and safeguarding patient safety. A well-structured PSMF is not merely a formality; it is essential for effective pharmacovigilance. It must incorporate:
- Detailed governance
- Designation of a qualified person for pharmacovigilance
- Establishment of robust documentation practices
These components are vital for maintaining data integrity and ensuring readiness for inspections. By adhering to these best practices, organizations can significantly enhance their pharmacovigilance efforts and contribute to overall patient safety.
Introduction
The pharmacovigilance system master file (PSMF) stands as a cornerstone of drug safety and regulatory compliance; however, many organizations grapple with its complexities. This vital document functions not only as a comprehensive repository for safety-related information but also as a reflection of the operational integrity of a Marketing Authorization Holder (MAH). As the landscape of pharmacovigilance evolves, a pressing question emerges: how can organizations effectively master the intricacies of the PSMF to ensure compliance and safeguard patient well-being? This article explores the essential steps for:
- Creating a robust PSMF
- Maintaining the PSMF
- Preparing for inspections of the PSMF
Thereby empowering stakeholders to enhance their pharmacovigilance practices.
Define the Pharmacovigilance System Master File (PSMF)
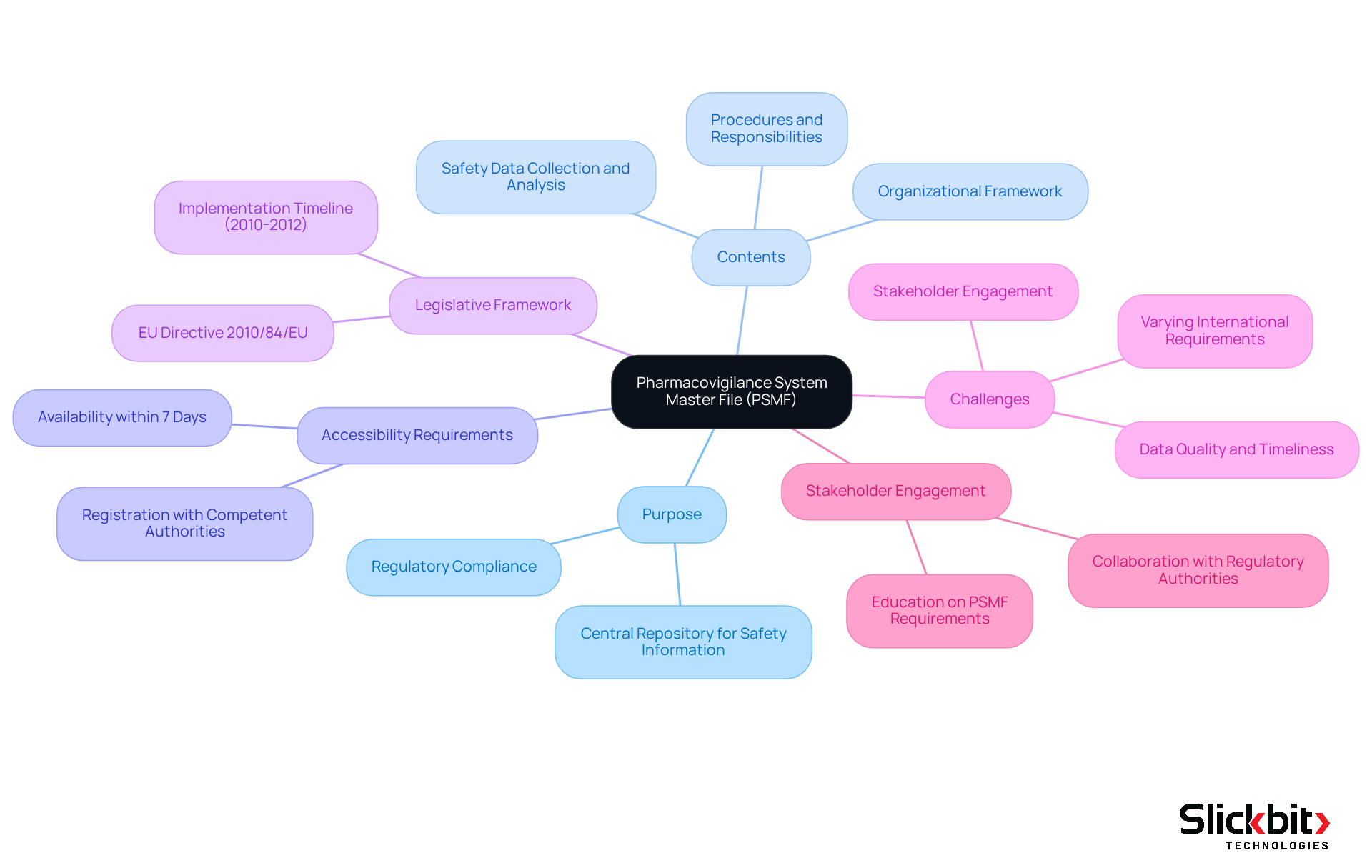
The pharmacovigilance system master file (PSMF) is a critical document that details the pharmacovigilance system utilized by a Marketing Authorization Holder (MAH). It acts as a central repository for all safety-related information concerning medicinal products, thereby playing a vital role in regulatory compliance. This document encompasses details about the organizational framework, procedures, and responsibilities associated with the pharmacovigilance system master file, ensuring that all safety data is systematically collected, analyzed, and recorded. Importantly, the relevant document must be accessible within seven days of a request from competent authorities or the European Medical Agency (EMA), underscoring the urgency of maintaining this file.
The legislative framework for the PSMF was established with the introduction of pertinent legislation in the EU in 2010 and its subsequent implementation in 2012, which delineates the regulatory expectations for MAHs. However, MAHs frequently encounter challenges due to differing international requirements, complicating the upkeep of their PSMFs. Engaging and informing stakeholders about these requirements is crucial for ensuring accurate data provision and compliance. Understanding the role of the pharmacovigilance system master file is essential for upholding regulatory standards and safeguarding patient well-being throughout the lifecycle of a drug product.
Prioritize Compliance and Strong PSMF Implementation
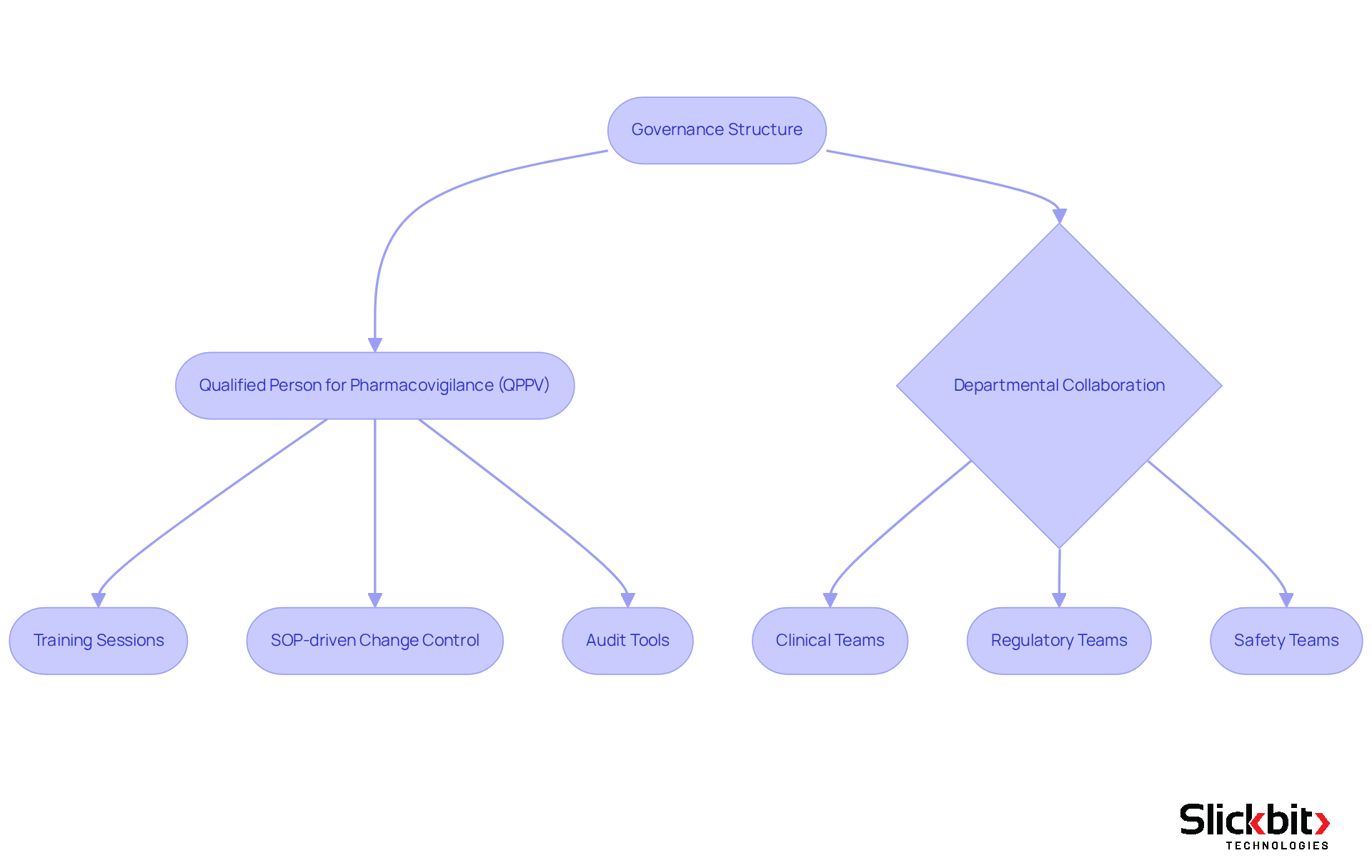
Creating a robust pharmacovigilance system master file is paramount for organizations, necessitating strict adherence to regulations such as the EU Pharmacovigilance Directive. This process commences with a clearly defined governance structure that allocates specific responsibilities to qualified personnel, including the Qualified Person for Pharmacovigilance (QPPV). Such measures ensure that all processes are meticulously documented and regularly reviewed.
Furthermore, successful implementation of this program hinges on collaboration among various departments—clinical, regulatory, and safety teams—to guarantee accurate capture and reporting of safety data. Consistent training sessions and updates regarding regulatory changes are crucial for maintaining compliance and adapting to evolving requirements.
In addition, linking updates to SOP-driven change control systems and utilizing audit tools to monitor inspection readiness and compliance metrics are essential best practices. By fostering a culture of continuous improvement and accountability, organizations can enhance their pharmacovigilance system master file practices, ensuring they remain prepared for inspections and reinforcing their commitment to patient well-being.
Identify Key Elements of a Compliant PSMF
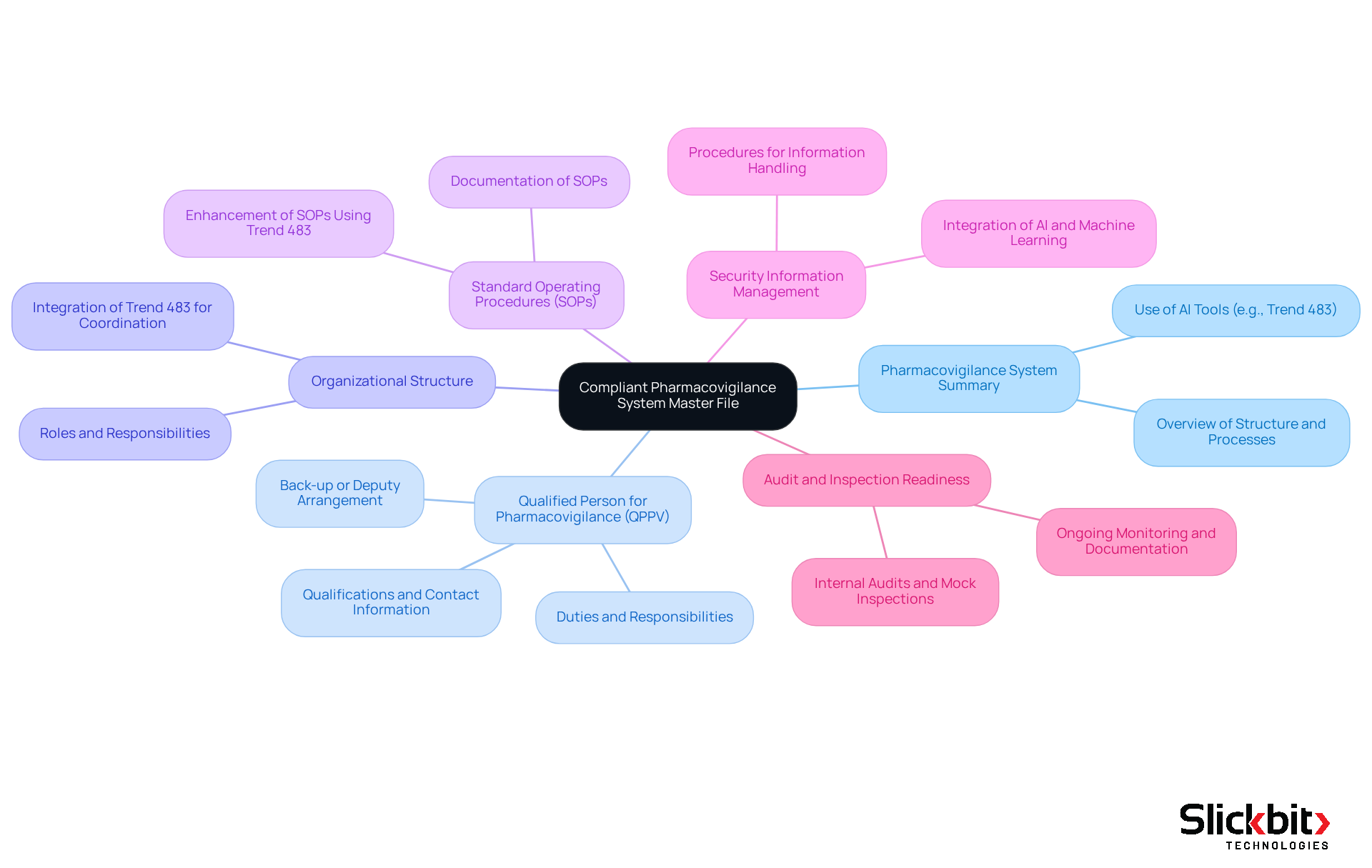
A compliant pharmacovigilance system master file must encompass several essential elements to ensure regulatory adherence and operational efficiency.
Summary of the Pharmacovigilance System: This section offers a thorough overview of the system's structure and processes, detailing how security information is handled and reported. Utilizing AI tools such as Slickbit's Trend 483 can enhance this process by identifying trends in systemic hazards and regulatory issues, thereby improving the overall management of risk data.
Qualified Person for Pharmacovigilance (QPPV): It is essential to incorporate comprehensive information regarding the QPPV's duties, qualifications, and contact information, as they play a key role in managing adherence and safety reporting. Additionally, a back-up or deputy for the QPPV should be designated to ensure continuous access to a medically qualified person.
Organizational Structure: A clear organizational chart should delineate the roles and responsibilities within the pharmacovigilance team, ensuring that all personnel understand their functions and reporting lines. The integration of Trend 483 can streamline these processes by providing insights that improve team coordination and adherence.
Standard Operating Procedures (SOPs): Documentation of all relevant SOPs governing pharmacovigilance activities is vital. These procedures direct the team in upholding regulations and ensuring consistent practices. Using Trend 483 can assist in the development and enhancement of these SOPs by identifying areas for improvement based on adherence trends.
Security Information Management: This section describes the procedures for gathering, examining, and reporting security information, highlighting the significance of precise and prompt information handling to protect patient health. The integration of technological advancements, such as AI and Machine Learning, should also be considered to enhance efficiency. Tools such as Trend 483 can offer deeper insights into safety data trends, further aiding effective data management and oversight monitoring.
Audit and Inspection Readiness: Evidence of ongoing monitoring and adherence checks must be documented to prepare for inspections. Regular internal audits and mock inspections are essential to ensure alignment with global and local pharmacovigilance practices. The legally mandated pharmacovigilance system master file, developed and upheld by the Marketing Authorization Holder (MAH), must accurately reflect the current condition of the pharmacovigilance system to meet regulatory requirements.
Sustaining these components in a thoroughly recorded and frequently refreshed way is essential for guaranteeing an adherent file, ultimately improving patient safety and regulatory adherence. Furthermore, ongoing dialogue between industry stakeholders and national regulatory authorities is vital for adapting to evolving regulations and ensuring compliance.
Prepare for PSMF Inspection Readiness
To ensure readiness for inspections of the pharmacovigilance system master file, organizations must adopt effective strategies that enhance compliance and operational excellence.
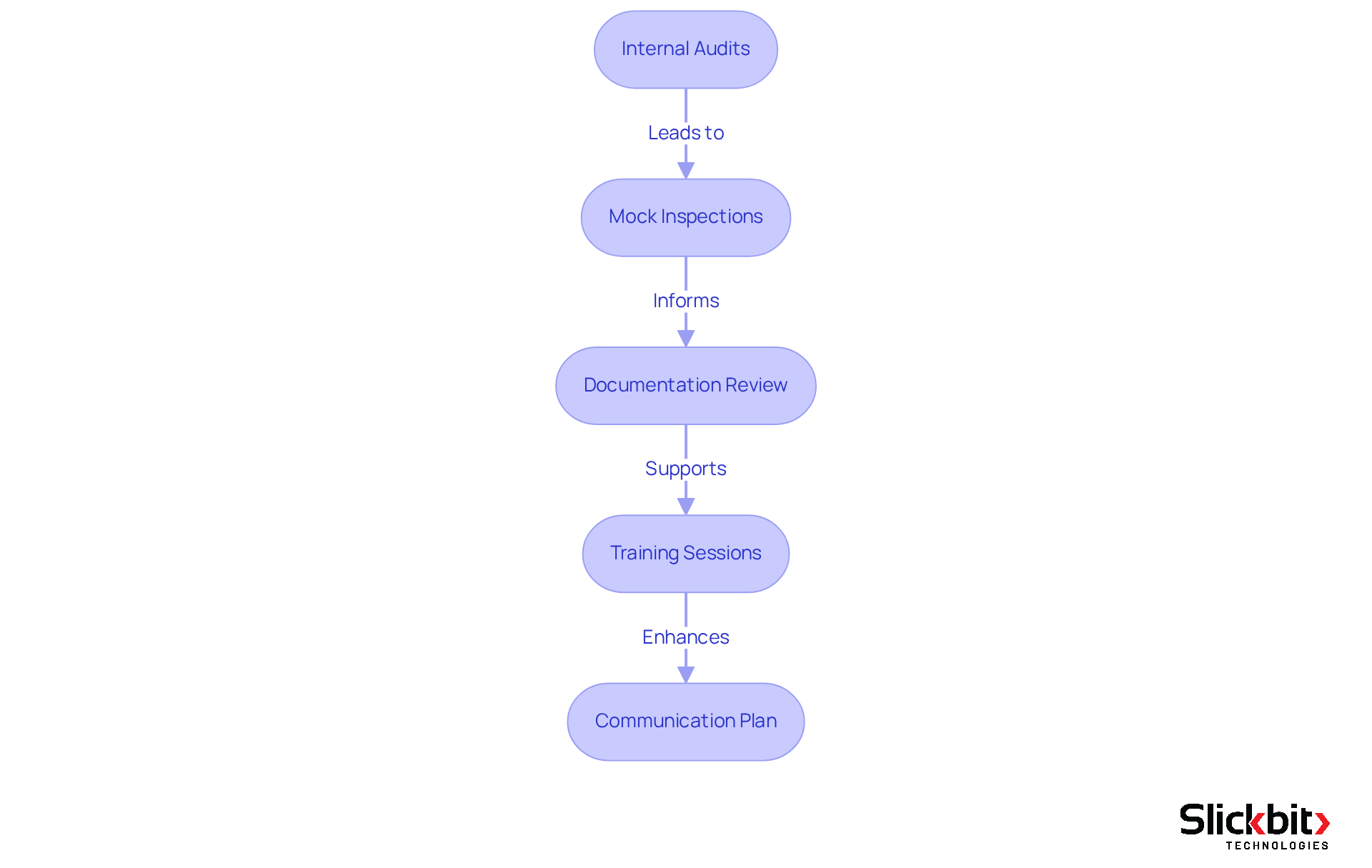
Regular internal audits of the pharmacovigilance system master file and related processes are crucial for identifying gaps and areas needing improvement. These audits should focus on adherence to regulatory standards and the accuracy of documentation within the pharmacovigilance system master file, ensuring that all practices align with industry expectations.
-
Mock Inspections: Simulating inspection scenarios enables teams to familiarize themselves with the inspection process and its expectations. Mock inspections have proven effective in identifying regulatory gaps and enhancing staff readiness, which is essential for compliance with the pharmacovigilance system master file, ultimately leading to improved outcomes during actual inspections.
-
Documentation Review: Maintaining all documents in an up-to-date and accurate state is essential. A centralized repository for the pharmacovigilance system master file facilitates quick retrieval, ensuring that all necessary information is readily accessible during inspections.
-
Training Sessions: Providing targeted training for staff on inspection protocols and their specific roles is vital. This training should include practical exercises that build confidence and ensure that team members are well-prepared for interactions with inspectors.
-
Communication Plan: Establishing a clear communication strategy is key to effective interactions with inspectors. Designating specific spokespersons can streamline communication and ensure consistent messaging throughout the inspection process.
By implementing these strategies, organizations can significantly enhance their inspection readiness, demonstrating a strong commitment to compliance and regulatory excellence. Engaging in regular mock inspections and internal audits not only prepares teams for scrutiny but also fosters a culture of continuous improvement in the pharmacovigilance system master file.
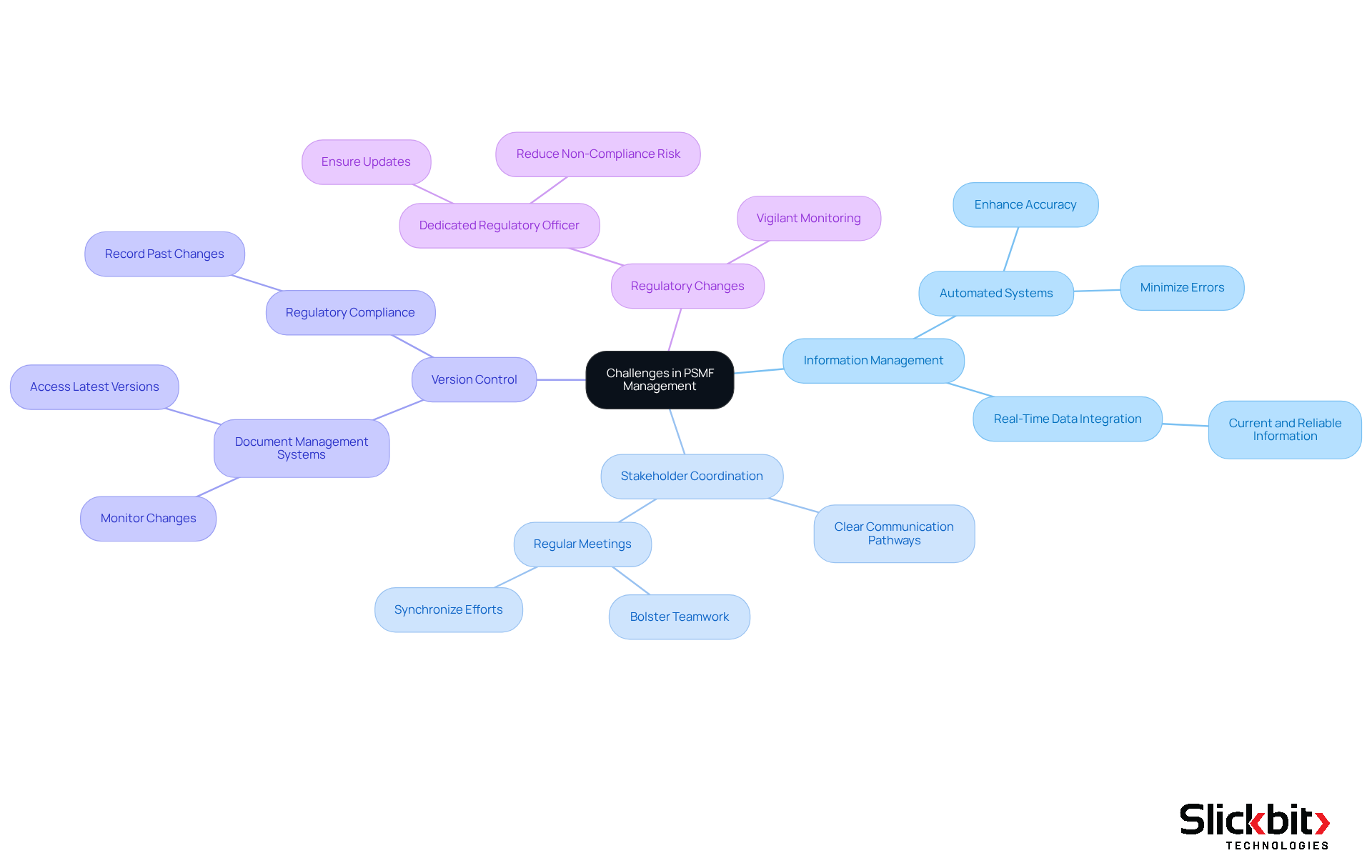
Address Challenges in PSMF Management
Managing a pharmacovigilance system master file presents several challenges that can significantly affect safety data integrity and compliance.
-
Information Management: The integrity and precision of security information are paramount, particularly when dealing with diverse information sources. Automated information management systems can streamline this process, enhancing accuracy and minimizing the risk of errors. These systems facilitate real-time data integration, ensuring that all safety information remains current and reliable.
-
Stakeholder Coordination: Efficient collaboration among various departments is essential to prevent delays in updates. Establishing clear communication pathways and organizing regular meetings can bolster teamwork, ensuring that all participants are synchronized and fully aware of their roles in the process.
-
Version Control: Clarity amidst multiple document versions can pose a challenge. Employing document management systems with robust version control capabilities enables organizations to effectively monitor changes, ensuring that the latest version is always accessible, while also recording past changes for regulatory purposes.
-
Regulatory Changes: The pharmacovigilance regulatory landscape is in constant flux, necessitating vigilant monitoring. Appointing a dedicated regulatory officer can help ensure that the Product Safety Management File is consistently updated to reflect current legal obligations, thereby reducing the risk of non-compliance.
By proactively addressing these challenges through the strategic implementation of automated systems and fostering collaboration, organizations can enhance their pharmacovigilance system master file management, thereby ensuring both data integrity and ongoing compliance.
Conclusion
The pharmacovigilance system master file (PSMF) is a critical document that underpins the safety and compliance of medicinal products. This comprehensive repository not only details the pharmacovigilance system of a Marketing Authorization Holder (MAH) but also ensures that safety-related information is meticulously managed and readily accessible to regulatory authorities. By grasping the significance of the PSMF, organizations can adeptly navigate the complexities of regulatory compliance while prioritizing patient safety.
Key insights from this guide underscore the necessity of a well-structured PSMF, which encompasses:
- A defined governance framework
- Clear roles and responsibilities
- Robust standard operating procedures
Furthermore, integrating innovative tools and practices—such as regular audits, mock inspections, and effective communication strategies—is essential for maintaining inspection readiness and ensuring adherence to evolving regulations. Addressing challenges like information management and stakeholder coordination further bolsters the integrity of the PSMF.
Ultimately, mastering the pharmacovigilance system master file transcends mere compliance; it signifies a commitment to safeguarding public health. Organizations are urged to adopt best practices and remain proactive in their pharmacovigilance approach, fostering a culture of continuous improvement. As regulatory landscapes evolve, the capacity to adapt and implement effective PSMF management strategies will be crucial in upholding the highest standards of drug safety and efficacy.
Frequently Asked Questions
What is the Pharmacovigilance System Master File (PSMF)?
The PSMF is a critical document that details the pharmacovigilance system used by a Marketing Authorization Holder (MAH). It serves as a central repository for all safety-related information concerning medicinal products and plays a vital role in regulatory compliance.
What information does the PSMF contain?
The PSMF includes details about the organizational framework, procedures, and responsibilities associated with the pharmacovigilance system, ensuring that all safety data is systematically collected, analyzed, and recorded.
How quickly must the PSMF be accessible to authorities?
The PSMF must be accessible within seven days of a request from competent authorities or the European Medical Agency (EMA).
When was the legislative framework for the PSMF established?
The legislative framework for the PSMF was established with the introduction of relevant legislation in the EU in 2010 and its implementation in 2012.
What challenges do Marketing Authorization Holders (MAHs) face regarding the PSMF?
MAHs often encounter challenges due to differing international requirements, complicating the maintenance of their PSMFs. Engaging and informing stakeholders about these requirements is crucial for ensuring accurate data provision and compliance.
Why is compliance important for the PSMF?
Compliance is essential for upholding regulatory standards and safeguarding patient well-being throughout the lifecycle of a drug product.
What are the key components for creating a robust PSMF?
Key components include a clearly defined governance structure, specific responsibilities allocated to qualified personnel such as the Qualified Person for Pharmacovigilance (QPPV), and meticulous documentation and regular review of processes.
How can organizations ensure successful implementation of the PSMF?
Successful implementation hinges on collaboration among clinical, regulatory, and safety teams, consistent training on regulatory changes, linking updates to SOP-driven change control systems, and utilizing audit tools to monitor compliance.
What practices can enhance PSMF practices within organizations?
Fostering a culture of continuous improvement and accountability, along with ensuring preparedness for inspections, can enhance PSMF practices and reinforce the organization's commitment to patient well-being.




