Overview
The article serves as a comprehensive guide for effectively navigating the 510(k) database search process for medical devices. It outlines detailed steps for conducting searches, troubleshooting common issues, and leveraging available resources. Understanding the 510(k) process is crucial, as it facilitates timely and successful submissions to the FDA. This guide empowers professionals in the pharmaceutical industry to enhance their submission strategies and optimize their search efforts.
Introduction
Navigating the complexities of the 510(k) process is crucial for medical device manufacturers striving to bring their innovations to market swiftly and effectively. This guide provides a comprehensive exploration of the 510(k) database search, emphasizing its importance in identifying predicate devices and streamlining applications.
As regulatory landscapes evolve and new challenges emerge, manufacturers must consider how to equip themselves to overcome common obstacles and leverage the latest tools for success. By understanding these dynamics, they can enhance their competitive edge and ensure compliance in a rapidly changing environment.
Understand the 510(k) Process and Its Importance
The 510(k) process serves as a crucial premarket application to the FDA, enabling manufacturers to demonstrate that their medical devices are both safe and effective by establishing substantial equivalence to existing products on the market. This pathway is particularly essential for Class I and Class II devices, allowing manufacturers to introduce their innovations without the extensive requirements associated with the Premarket Approval (PMA) process.
A comprehensive understanding of the intricacies involved in the 510(k) database search process is vital for ensuring compliance and facilitating timely market entry for new medical devices. Regulatory specialists emphasize that a thorough grasp of this process, particularly the 510(k) database search, empowers manufacturers to:
- Identify suitable predicate devices
- Streamline their applications
- Minimize common obstacles that could lead to approval delays
Companies adept at navigating the 510(k) database search can significantly accelerate their time to market; recent trends indicate that the FDA reviews approximately 3,000 510(k) applications annually, with an average review time of around 90 days.
Furthermore, recent updates from the FDA, including the mandatory use of the eSTAR format for applications beginning October 1, 2023, underscore the importance of staying informed about regulatory changes. By proactively engaging with these requirements, manufacturers can enhance their chances of successful proposals and ultimately deliver innovative medical solutions to patients more efficiently.
Additionally, leveraging AI tools, such as Trend 483, offers valuable insights into systemic risks and compliance trends derived from FDA inspections. Such insights can assist manufacturers in streamlining their 510(k) submissions by utilizing a 510(k) database search to identify potential compliance issues early in the process, thereby improving operational efficiency and informed decision-making.
Follow the Step-by-Step Search Process for 510(k) Database
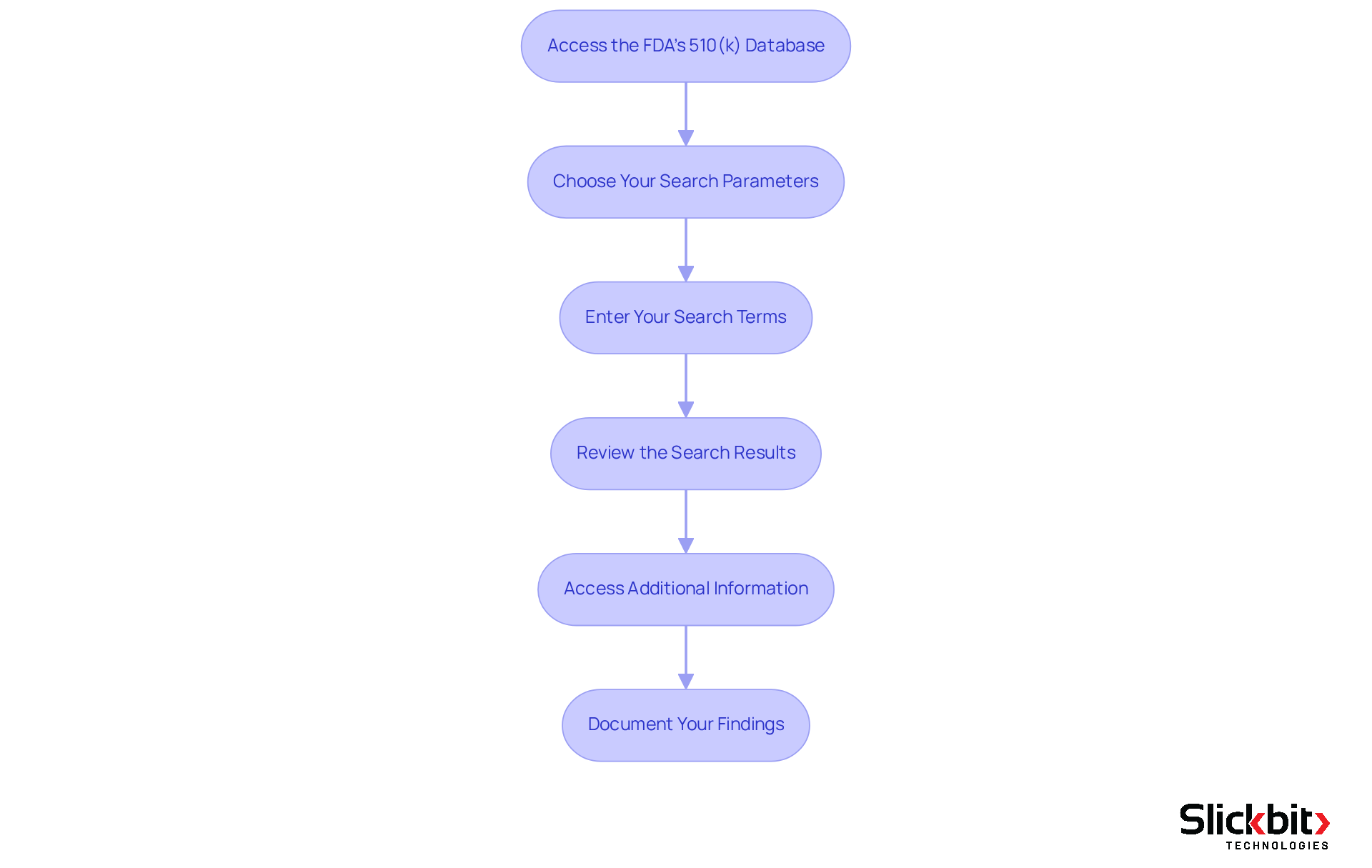
To effectively search the 510(k) database, follow these essential steps:
-
Access the FDA's 510(k) Database: Begin by visiting the FDA's official website and navigating to the 510(k) section under 'Medical Devices.' This area contains the searchable database crucial for your research.
-
Choose Your Search Parameters: You can look up using various criteria, including the 510(k) number, product code, device name, or applicant name. Select the criteria that best align with your needs.
-
Enter Your Search Terms: Input your selected criteria into the inquiry fields. For instance, if you are searching for a specific device, enter its name or the relevant product code to refine your results.
-
Review the Search Results: After submitting your search, carefully review the results. Each entry will provide essential details about the device, including its clearance status and any associated documents.
-
Access Additional Information: Click on the relevant entries to access more detailed information, including summaries of the 510(k) applications and guidance documents that can assist in understanding the device's regulatory pathway.
-
Document Your Findings: Keep a record of the devices you uncover, especially those that may function as predicate devices for your entries. This documentation is invaluable for your regulatory strategy, as 85% of 510(k) rejections stem from issues related to demonstrating substantial equivalence. Furthermore, it is crucial to prepare thoroughly, as 32 percent of FDA 510(k) applications failed the acceptance for review check in the year up to September 2022. Engaging with experts, such as Ana Criado, who offers invaluable insights into this process, can significantly enhance your understanding and preparation.
By mastering these steps, you can efficiently navigate the 510(k) database search, which will enhance your chances of successful submissions and expedite your product development timelines. Remember, the FDA clears approximately 3,000 medical devices for marketing via the 510(k) pathway each year, making this database a vital resource in the medical device market.
Troubleshoot Common Issues in 510(k) Database Searches
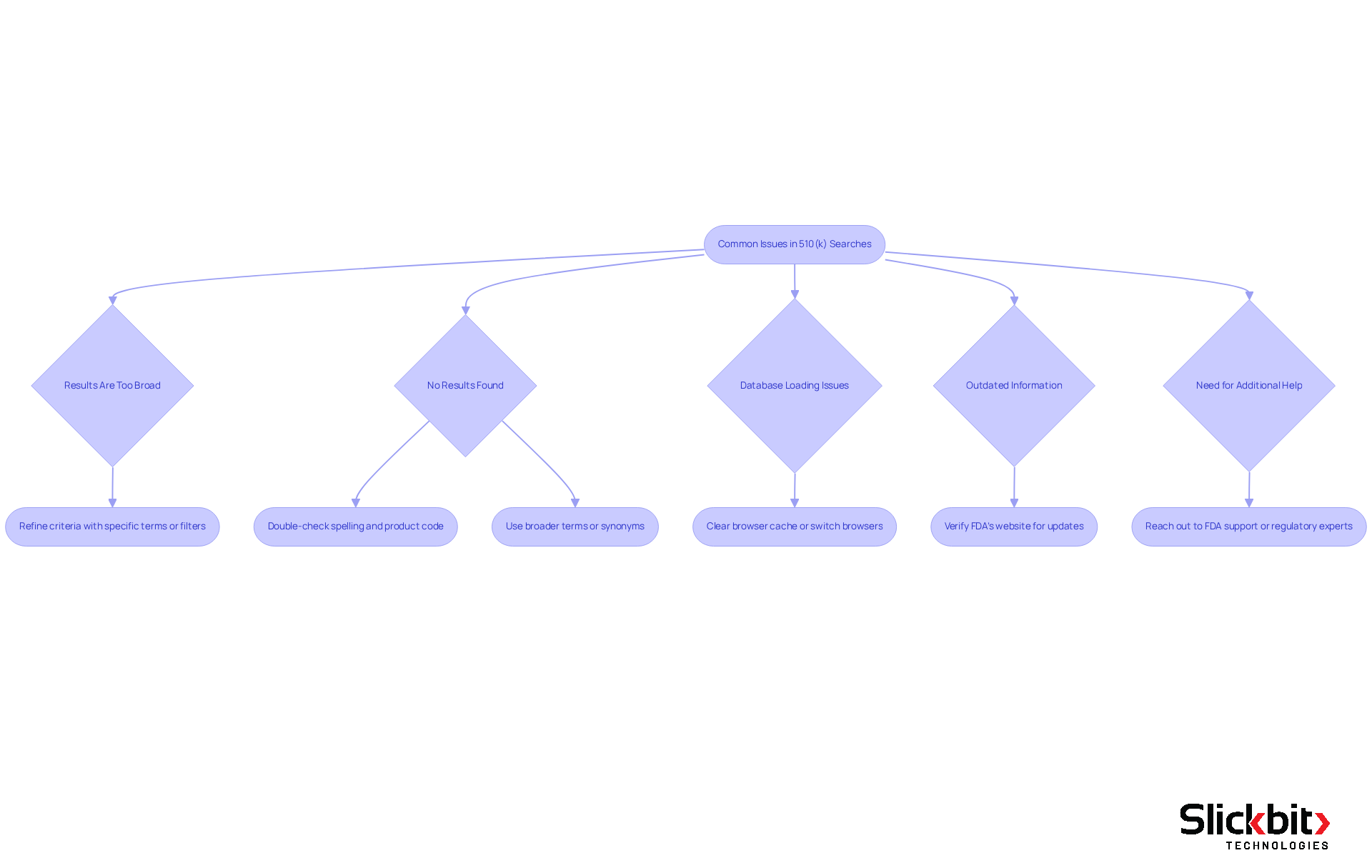
While conducting a 510 k database search can be straightforward, users may encounter common issues that require attention. Here are some troubleshooting tips to help you navigate these challenges effectively:
-
Results Are Too Broad: If your inquiry yields an overwhelming number of outcomes, refine your criteria by incorporating more specific terms or utilizing the filters available within the database. Approximately 60% of users report facing extensive results, highlighting this as a prevalent obstacle.
-
No Results Found: Should your inquiry yield no results, it is crucial to double-check your spelling and ensure that you are using the correct product code or device name. Additionally, consider employing broader terms or synonyms to enhance your search.
-
Database Loading Issues: In instances where the database is slow to load or unresponsive, attempt clearing your browser cache or switching to a different browser. Technical issues can occasionally impede access, so this step may resolve the problem.
-
Outdated Information: If you suspect that the information may be outdated, verify the FDA's website for any recent updates or announcements regarding the database or specific devices. Staying informed is essential for conducting precise inquiries.
-
Need for Additional Help: If challenges persist, consider reaching out to the FDA's support or consulting with regulatory experts who can provide guidance on effectively navigating the database. Regulatory advisors emphasize the importance of leveraging expert insights to overcome typical challenges.
Utilize Tools and Resources for Effective Searching
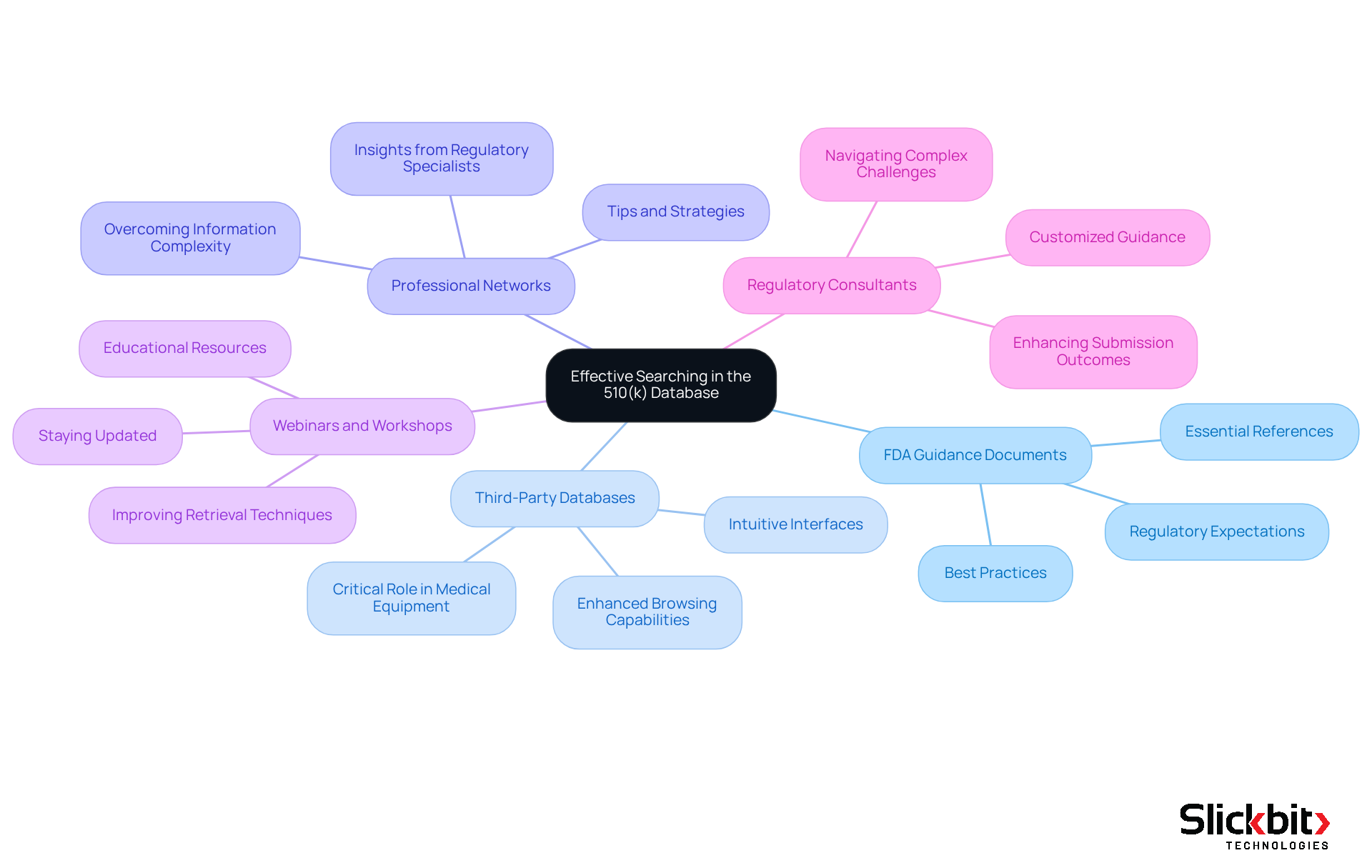
To enhance your search capabilities within the 510(k) database, consider utilizing the following tools and resources:
-
FDA Guidance Documents: Familiarize yourself with the latest FDA guidance documents concerning 510(k) applications. These documents outline best practices and regulatory expectations, serving as essential references for successful submissions. Understanding these guidelines is crucial for navigating the complexities of the application process.
-
Third-Party Databases: Explore third-party databases that compile FDA data, offering enhanced browsing capabilities and intuitive interfaces. These resources can significantly simplify the retrieval process and enhance efficiency. Notably, approximately 99% of products authorized for human use are cleared through the 510(k) database, underscoring its critical role in the medical equipment sector.
-
Professional Networks: Engage with professional networks and forums where regulatory specialists share insights and experiences. These platforms provide valuable tips and strategies for effective searching, enhancing your understanding of the regulatory landscape. The complexity of synthesizing information from various sources can pose challenges, making these networks invaluable for professionals seeking clarity.
-
Webinars and Workshops: Attend webinars and workshops focused on FDA submissions and regulatory strategies. These educational resources can deepen your understanding and improve your retrieval techniques, ensuring you stay updated on the latest practices. Recognizing that the average time to obtain an FDA 510(k) decision is approximately five months emphasizes the urgency of employing effective 510(k) database search strategies.
-
Regulatory Consultants: If you encounter intricate challenges or require customized guidance, consider seeking assistance from regulatory specialists who focus on 510(k) filings. Their expertise can help navigate the nuances of the process, ultimately enhancing outcomes. As highlighted by experts like Ana Criado and Katherine Ruiz, leveraging FDA guidance documents is essential for successful submissions.
Conclusion
Mastering the 510(k) database search is essential for manufacturers aiming to navigate the complexities of medical device regulation effectively. Understanding the significance of the 510(k) process and employing a systematic approach to database searches can significantly enhance manufacturers' chances of timely approval and market entry. The insights shared throughout this guide provide a roadmap for successfully engaging with the FDA's requirements and optimizing the submission process.
Key points discussed include:
- The importance of identifying suitable predicate devices
- Utilizing effective search parameters
- Leveraging available tools and resources
- Troubleshooting common search issues
- Engaging with regulatory experts
These are vital strategies for overcoming obstacles and ensuring compliance. Furthermore, incorporating AI tools and staying informed about regulatory updates, such as the mandatory eSTAR format, can further streamline the submission process.
Ultimately, the ability to conduct a thorough 510(k) database search not only facilitates compliance but also drives innovation in the medical device industry. As the landscape continues to evolve, manufacturers are encouraged to embrace these best practices and utilize the wealth of resources available. By doing so, they can position themselves for success, ensuring that new medical solutions reach patients in a timely and efficient manner.
Frequently Asked Questions
What is the 510(k) process?
The 510(k) process is a premarket application to the FDA that allows manufacturers to demonstrate that their medical devices are safe and effective by establishing substantial equivalence to existing products on the market.
Why is the 510(k) process important?
The 510(k) process is essential for Class I and Class II devices, enabling manufacturers to introduce their innovations without the extensive requirements associated with the Premarket Approval (PMA) process.
What are the benefits of understanding the 510(k) database search process?
A comprehensive understanding of the 510(k) database search process helps manufacturers identify suitable predicate devices, streamline their applications, and minimize common obstacles that could lead to approval delays.
How many 510(k) applications does the FDA review annually?
The FDA reviews approximately 3,000 510(k) applications annually.
What is the average review time for a 510(k) application?
The average review time for a 510(k) application is around 90 days.
What recent update has the FDA made regarding the 510(k) process?
The FDA has mandated the use of the eSTAR format for applications starting October 1, 2023.
How can manufacturers enhance their chances of successful proposals in the 510(k) process?
By proactively engaging with regulatory requirements and staying informed about changes, manufacturers can enhance their chances of successful proposals.
What role do AI tools play in the 510(k) process?
AI tools, such as Trend 483, provide valuable insights into systemic risks and compliance trends from FDA inspections, helping manufacturers streamline their 510(k) submissions and identify potential compliance issues early in the process.




