Overview
This article serves as a comprehensive guide for R&D managers seeking to master the 510(k) database, highlighting its critical role in regulatory compliance, market research, and efficient product development. Understanding the intricacies of this database is essential for navigating the complexities of medical equipment approvals.
The article meticulously outlines the steps for:
- Determining eligibility
- Preparing submissions
- Overcoming common challenges
Thereby illustrating that effective utilization of the 510(k) database can significantly enhance the chances of successful product approvals within the medical equipment sector.
Introduction
Navigating the complex landscape of medical device regulation can be daunting for R&D managers eager to bring innovative products to market. The 510(k) database serves as a pivotal resource in this journey, offering essential insights into FDA-approved devices that can significantly influence product development strategies and ensure compliance.
However, the intricacies surrounding eligibility, documentation, and submission processes present considerable challenges for many managers.
How can they effectively leverage the 510(k) database to streamline their path to market success while avoiding common pitfalls? This inquiry is crucial for those seeking to navigate the regulatory maze with confidence.
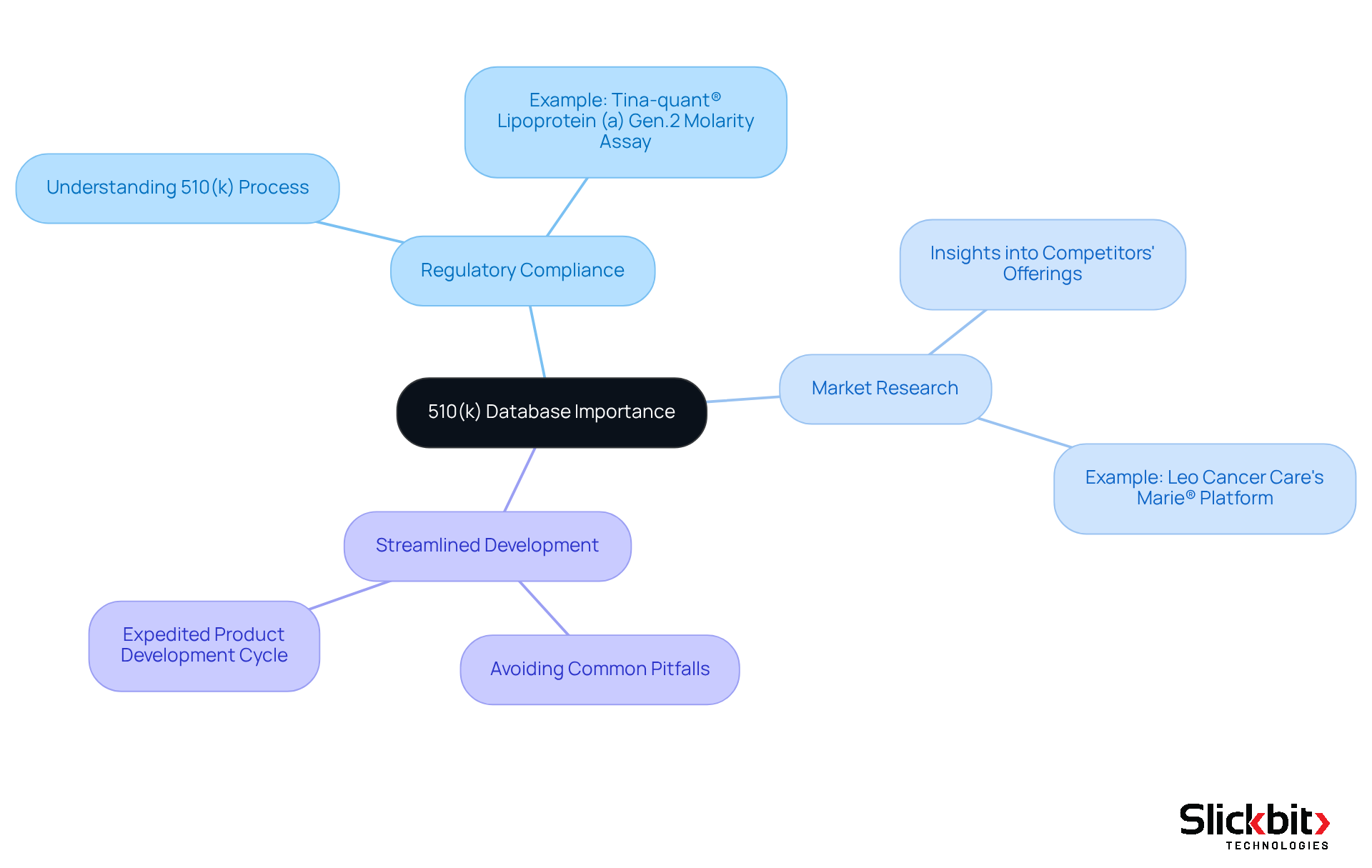
Understand the 510(k) Database and Its Importance
The 510 k database serves as a vital resource for R&D managers in the medical equipment sector, offering essential information on products that have been approved for marketing by the FDA. This resource is indispensable for several reasons:
-
Regulatory Compliance: A comprehensive understanding of the 510(k) process is critical for ensuring that your product meets FDA standards, significantly reducing the risk of delays or rejections. For example, the recent FDA clearance of the Tina-quant® Lipoprotein (a) Gen.2 Molarity assay demonstrates how compliance can facilitate market entry for innovative products.
-
Market Research: The database delivers valuable insights into competitors' offerings, enabling you to discern market trends and identify potential gaps. Companies like Leo Cancer Care have effectively leveraged the 510 k database to inform their product development strategies, as evidenced by their upright radiotherapy platform, Marie®, which recently received FDA clearance.
-
Streamlined Development: Acquaintance with the 510(k) requirements and common pitfalls can dramatically expedite your product development cycle. By utilizing the database, R&D teams can sidestep missteps that could result in costly delays.
Mastering the 510 k database equips R&D managers to navigate the regulatory landscape with assurance, ensuring that their products not only comply with standards but also achieve efficient and effective market entry.
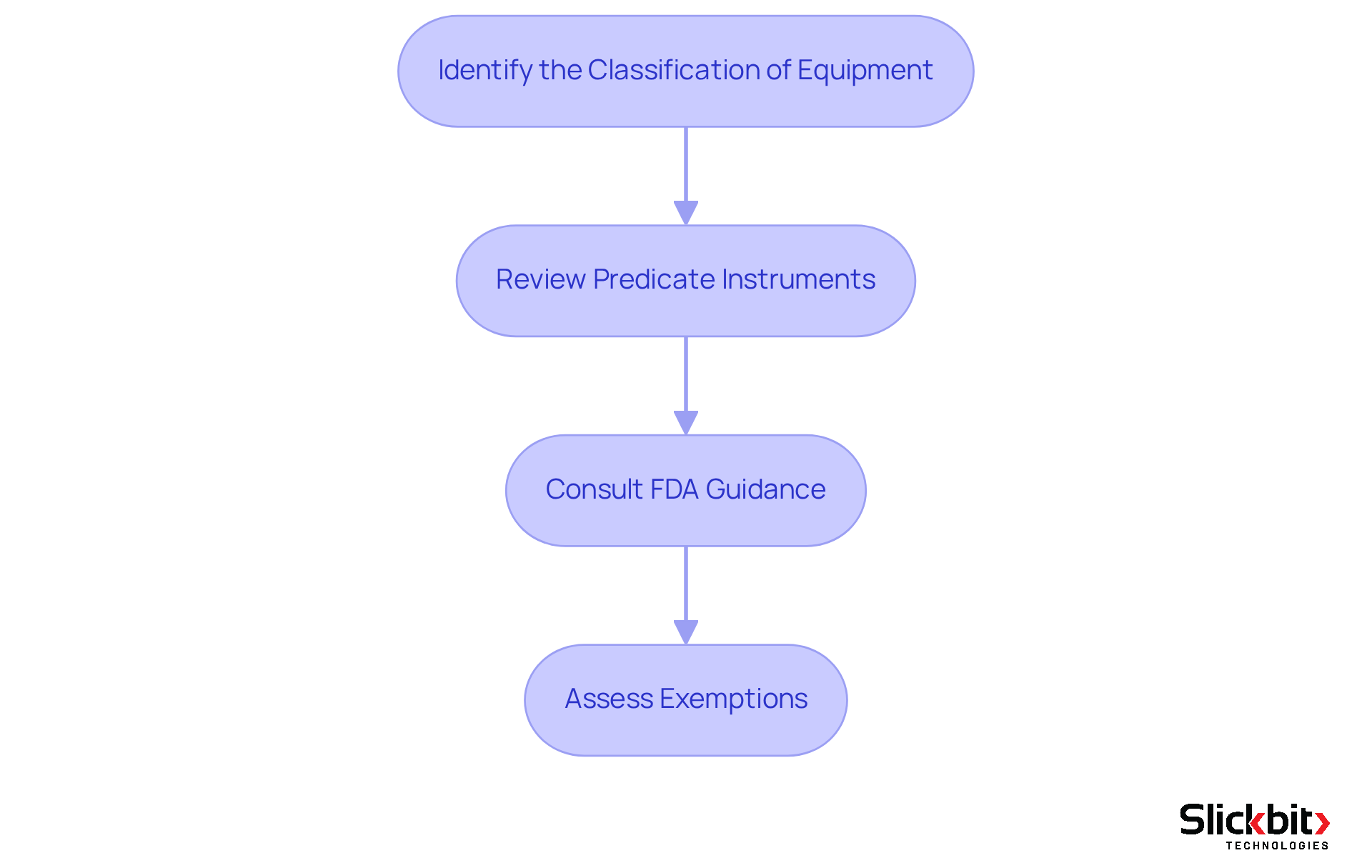
Determine Eligibility for 510(k) Submission
To determine your device's eligibility for a 510(k) submission, follow these essential steps:
- Identify the Classification of Equipment: Begin by checking whether your equipment falls under Class I, II, or III. Notably, the majority of submissions in the 510 k database pertain to Class II instruments.
- Review Predicate Instruments: Ensure that your product is substantially equivalent to a legally marketed predicate instrument. This equivalence entails having the same intended use and technological characteristics.
- Consult FDA Guidance: Utilize the FDA's guidance documents to gain insight into the specific requirements applicable to your product type.
- Assess Exemptions: Certain items may be exempt from 510(k) requirements. Confirm whether your equipment qualifies for any such exemptions.
By thoroughly assessing these factors, you can effectively ascertain your apparatus's eligibility for the 510 k database, thereby conserving both time and resources.
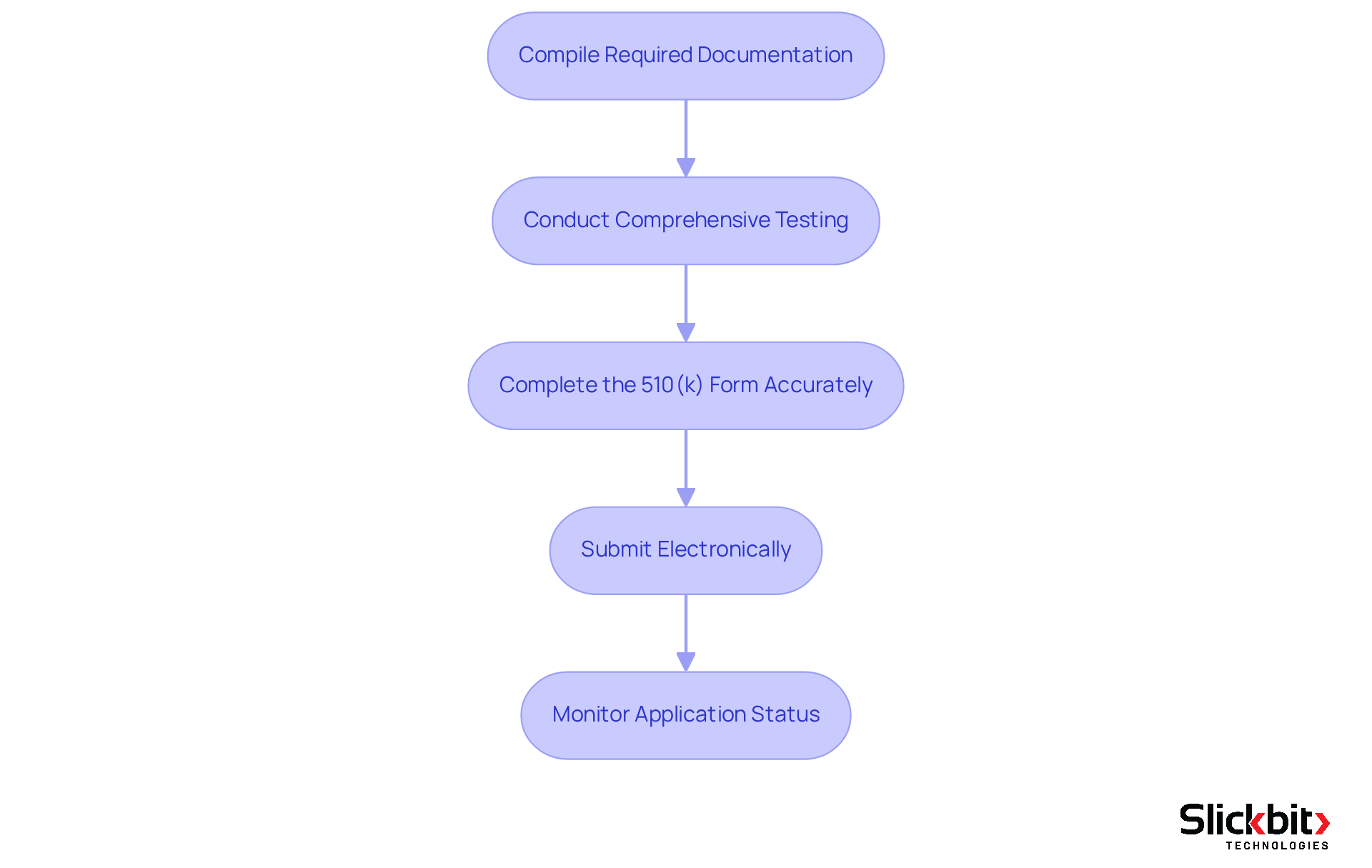
Prepare and Submit Your 510(k) Application
Preparing and submitting your application to the 510 k database demands meticulous attention to detail and strict adherence to regulatory standards. To ensure a successful submission, follow these essential steps:
-
Compile Required Documentation: Gather all necessary documents, including a detailed device description, intended use, labeling, and performance data. Address all sections of the eSTAR template, even if some are not applicable, to avoid delays. Organizations that emphasize documentation quality have seen their success rates rise, with the FDA indicating a 95% success rate for thoroughly prepared entries.
-
Conduct Comprehensive Testing: Perform all required testing to demonstrate safety and effectiveness, including biocompatibility assessments and performance evaluations. Comprehensive performance testing is crucial for establishing substantial equivalence, and presenting this data clearly in the 510 k database can significantly enhance the understanding of reviewers.
-
Complete the 510(k) Form Accurately: Fill out the FDA's 510(k) application form with precision, ensuring that all sections are complete. A well-structured entry reflects professionalism and can facilitate a smoother review process. Remember, the foundation of every successful application in the 510 k database lies in selecting the appropriate predicate device, as emphasized by regulatory experts.
-
Submit Electronically: Utilize the FDA's electronic application portal to send your request. This method simplifies the process and provides confirmation of receipt, which is crucial for monitoring your entry.
-
Monitor Application Status: After submission, actively track the status of your application through the FDA's database. Engage with the FDA early through pre-submissions to identify potential issues and respond promptly to any requests for additional information. Timely communication can prevent unnecessary delays. Furthermore, be mindful of the costs linked to the 510 k database applications, including the standard user fee of $25,952 and potential expenses for consultants, which can significantly influence your budget.
By diligently adhering to these procedures and addressing potential issues, such as insufficient documentation or inadequate testing, you can greatly enhance the likelihood of a successful 510(k) filing. Organizations that prioritize documentation quality have experienced a rise in success rates, with the FDA indicating a 95% success rate for well-prepared entries.
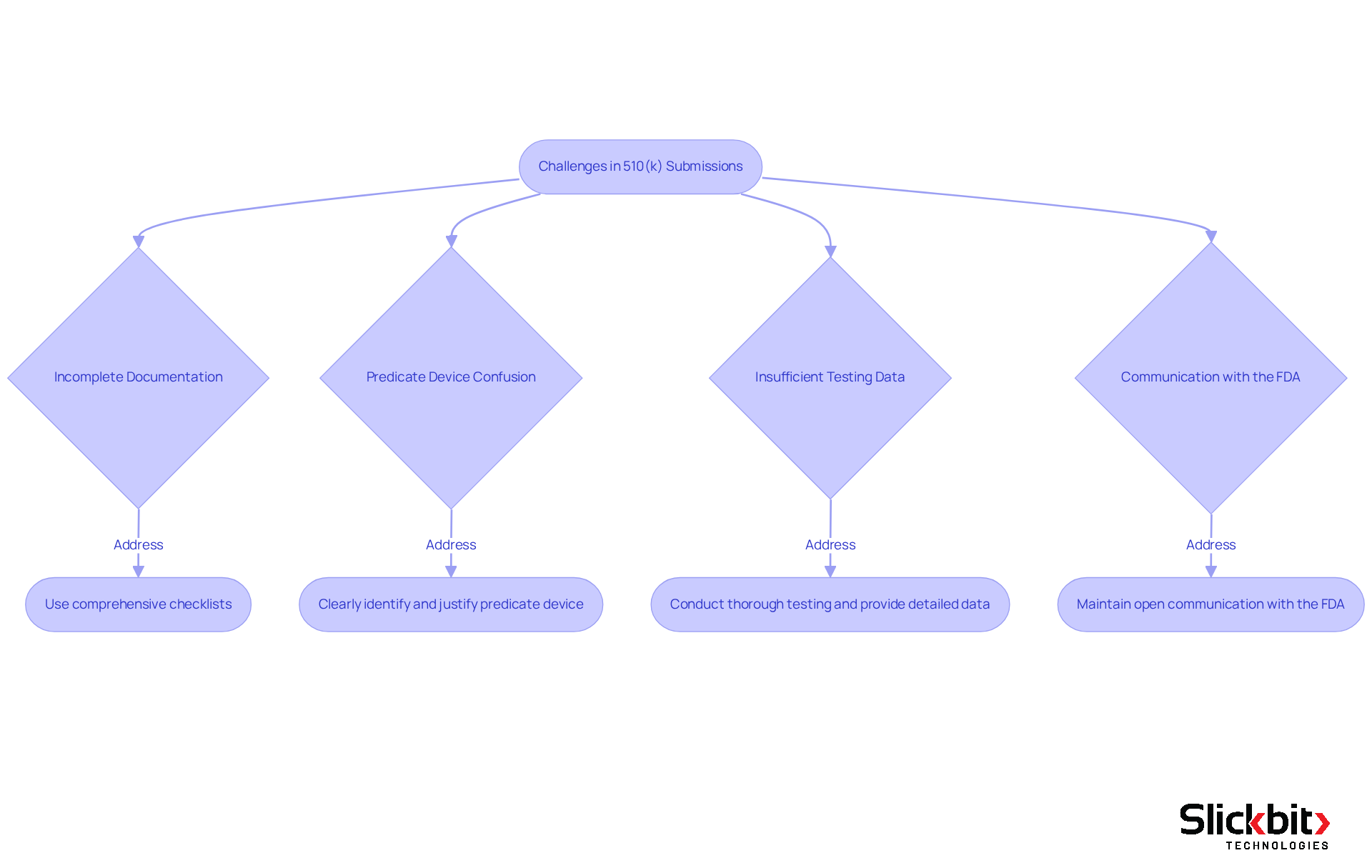
Overcome Common Challenges in 510(k) Submissions
Navigating the 510 k database filing process presents several challenges that can impede success. Common issues include incomplete documentation, predicate device confusion, insufficient testing data, and communication with the FDA. Addressing these challenges effectively is crucial for a successful submission.
-
Incomplete Documentation: Nearly 20 percent of 510(k) entries face delays due to incomplete documentation. To mitigate this risk, ensure that all required documents are complete and accurate. Utilizing comprehensive checklists serves as an effective strategy to verify that no detail is overlooked.
-
Predicate Device Confusion: It is essential to clearly identify and justify the predicate device in your documentation. Misunderstandings in this area can lead to rejection. Therefore, selecting predicate devices that are relevant and cleared using well-established methods is crucial for your submission's success.
-
Insufficient Testing Data: Robust testing data is vital to support your claims effectively. Conduct thorough testing and provide detailed data to substantiate your findings. Engaging with regulatory experts can further validate your results, enhancing the credibility of your presentation.
-
Communication with the FDA: Maintaining open lines of communication with the FDA is imperative. If you receive feedback, address it promptly and comprehensively. This demonstrates your commitment to compliance and quality, reinforcing your dedication to the process.
By anticipating these challenges and preparing accordingly, R&D managers can significantly enhance their chances of a successful submission to the 510 k database.
Conclusion
Mastering the 510(k) database is not merely a regulatory necessity; it is a strategic advantage for R&D managers in the medical equipment industry. This invaluable resource serves as a roadmap for navigating the complexities of regulatory compliance and market entry. By understanding FDA standards and leveraging insights from the 510(k) database, R&D teams can significantly enhance their chances of successful product launches while ensuring adherence to regulatory requirements.
Key arguments presented in this guide underscore the importance of:
- Determining eligibility for 510(k) submission
- Preparing thorough applications
- Effectively overcoming common challenges
From identifying the correct classification of equipment to compiling comprehensive documentation and maintaining effective communication with the FDA, each step is crucial in achieving a successful submission. Moreover, the guide emphasizes the significance of proactive measures, such as consulting FDA guidance and utilizing checklists, to avoid potential pitfalls.
Ultimately, embracing the 510(k) database empowers R&D managers to not only meet market demands but also drive innovation in the medical device sector. By prioritizing thorough preparation and addressing potential challenges head-on, organizations can significantly enhance their success in navigating the regulatory landscape and bringing life-saving technologies to market. It is imperative that R&D managers fully utilize this resource to contribute meaningfully to the advancement of healthcare solutions.
Frequently Asked Questions
What is the 510(k) database?
The 510(k) database is a resource that provides essential information on medical products that have been approved for marketing by the FDA, serving as a vital tool for R&D managers in the medical equipment sector.
Why is the 510(k) database important for regulatory compliance?
Understanding the 510(k) process is critical for ensuring that products meet FDA standards, which significantly reduces the risk of delays or rejections during the approval process.
How can the 510(k) database assist in market research?
The database offers valuable insights into competitors' products, helping companies identify market trends and potential gaps, which can inform their product development strategies.
In what way does the 510(k) database streamline product development?
Familiarity with the 510(k) requirements and common pitfalls can expedite the product development cycle, allowing R&D teams to avoid costly missteps and delays.
How does mastering the 510(k) database benefit R&D managers?
Mastering the 510(k) database enables R&D managers to navigate the regulatory landscape confidently, ensuring their products comply with standards and achieve efficient market entry.




