Overview
This article presents a comprehensive step-by-step guide for R&D managers engaged in medical device development. It underscores critical areas such as:
- Market trends
- Regulatory compliance
- Technological innovations
- Cross-functional collaboration
By outlining essential phases in the development process, the article offers strategies for:
- Ensuring compliance and quality assurance
- Overcoming common challenges
Consequently, it equips managers with the necessary knowledge to navigate the complexities of the medical device landscape effectively.
Introduction
Navigating the intricate landscape of medical device development presents a distinctive array of challenges and opportunities for R&D managers. As the industry advances rapidly, driven by technological innovations and stringent regulatory requirements, grasping the nuances of this field becomes essential. This article explores the critical phases of medical device development, emphasizing key strategies that empower R&D leaders to bolster collaboration, ensure compliance, and ultimately drive innovation.
What are the most pressing obstacles that R&D teams face? How can they effectively overcome these challenges to thrive in this competitive market?
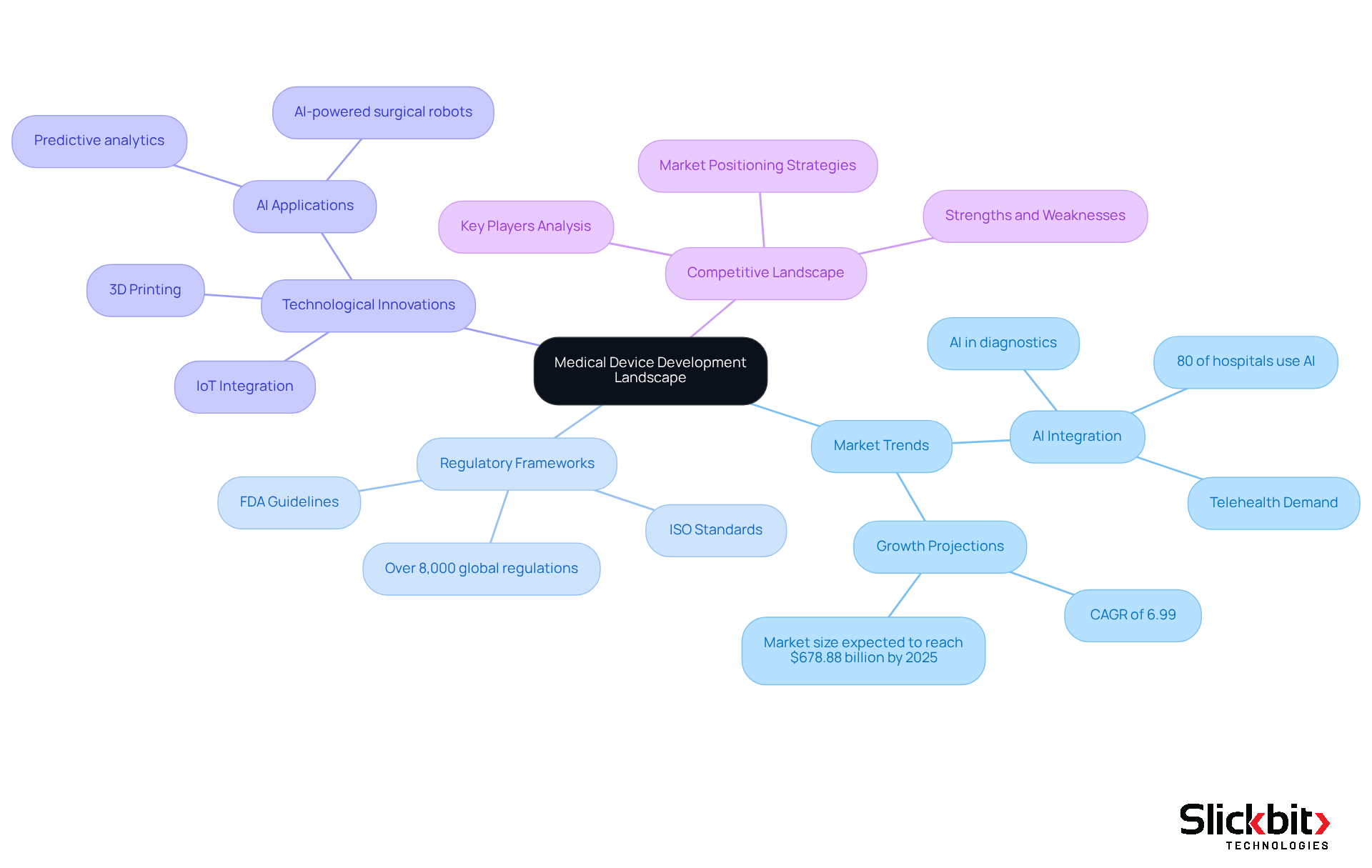
Understand the Medical Device Development Landscape
To effectively navigate the medical device development landscape, R&D managers must prioritize several critical areas:
-
Market Trends: It is essential to analyze the current trends in medical technology, particularly the growing integration of AI in diagnostics and the rising demand for telehealth solutions. Notably, 80% of hospitals now utilize AI to enhance patient care and operational efficiency, reflecting a significant shift towards technology-driven healthcare.
-
Familiarity with the regulatory frameworks governing medical device development, including FDA guidelines and ISO standards, is crucial. With over 8,000 global regulations, understanding these requirements is vital for ensuring compliance throughout medical device development and avoiding costly delays.
-
Technological Innovations: Staying informed about emerging technologies that can enhance functionality is imperative. Advanced materials, IoT integration, and AI applications are at the forefront of innovation. For instance, AI-powered systems are increasingly utilized in hospitals to analyze medical images, improving diagnostic accuracy and enabling timely interventions.
-
Competitive Landscape: Identifying key players in the market and analyzing their product offerings, strengths, and weaknesses is essential. This competitive analysis will guide your strategy and assist in positioning your product effectively. As the medical equipment industry is anticipated to expand at a CAGR of 6.99%, comprehending the competitive dynamics will be vital for success.
By diligently collecting this information, R&D managers can establish a robust foundation for their medical device development projects, ensuring alignment with industry standards and market needs.
Explore the Phases of Medical Device Development
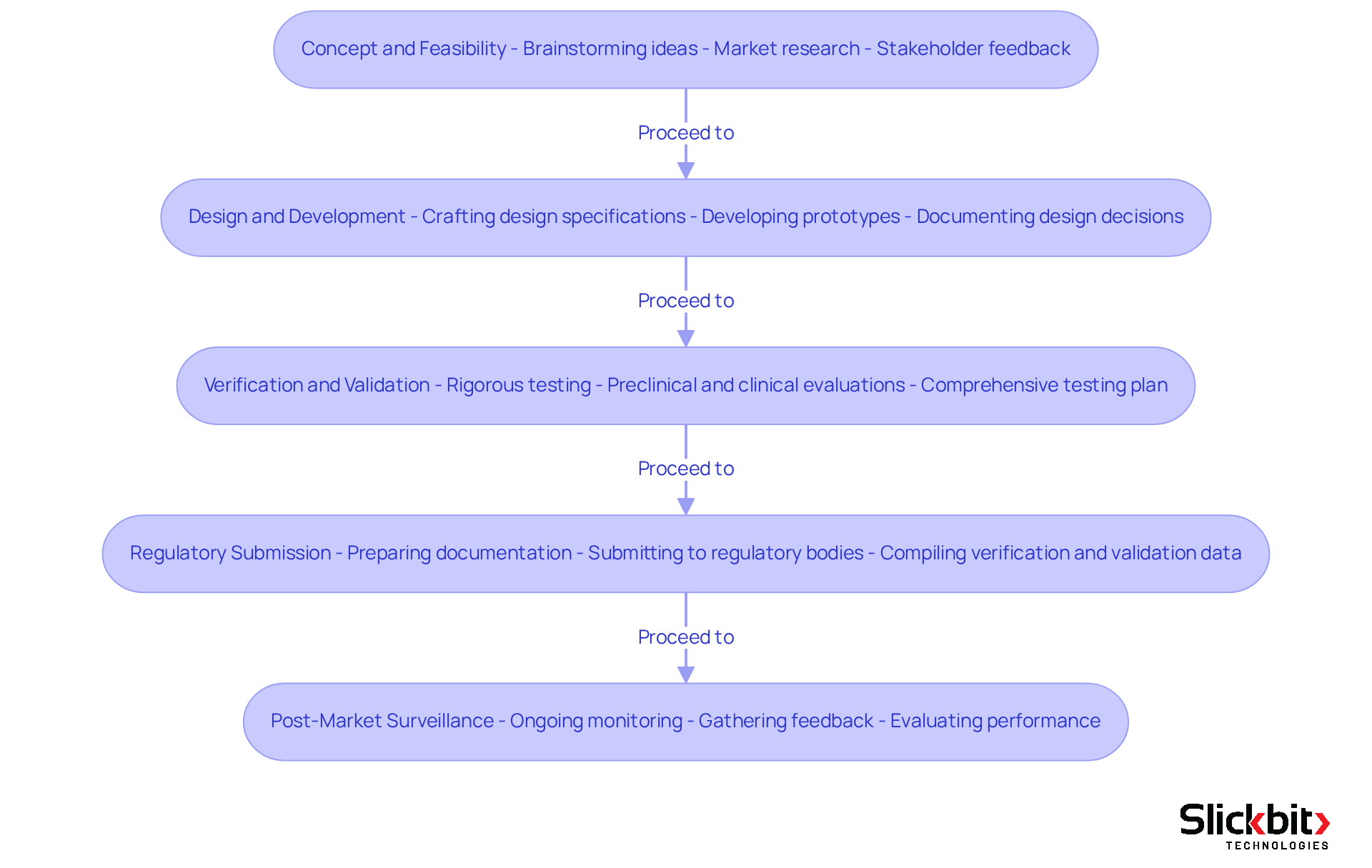
The medical device development process typically consists of several critical phases that are essential for successful outcomes:
- Concept and Feasibility: This initial phase is pivotal, involving the brainstorming of innovative ideas, conducting thorough market research, and evaluating the practicality of the proposed apparatus. R&D supervisors should actively engage stakeholders to collect valuable feedback, thereby enhancing the concept.
- Design and Development: During this phase, detailed design specifications are meticulously crafted, and prototypes are developed. It is crucial to document design decisions and maintain a Design History File (DHF) to ensure compliance with regulatory standards.
- Verification and Validation: This phase encompasses rigorous testing to verify that the device meets established design specifications and validation to confirm that it fulfills its intended use. R&D supervisors are tasked with creating a comprehensive testing plan that includes both preclinical and clinical evaluations.
- Regulatory Submission: Following the completion of testing, R&D supervisors must prepare and submit the necessary documentation to regulatory bodies for approval. This process includes compiling data from the verification and validation phases to demonstrate the device's safety and efficacy.
- Post-Market Surveillance: After the product launch, ongoing monitoring becomes essential to ensure continued compliance and to gather feedback for future enhancements. R&D supervisors ought to establish a robust post-market monitoring strategy to evaluate performance and address any issues that may arise.
By comprehending these stages, R&D leaders can effectively guide their teams through the creation process, ensuring timely and compliant product launches.
Ensure Compliance and Quality Assurance Throughout Development
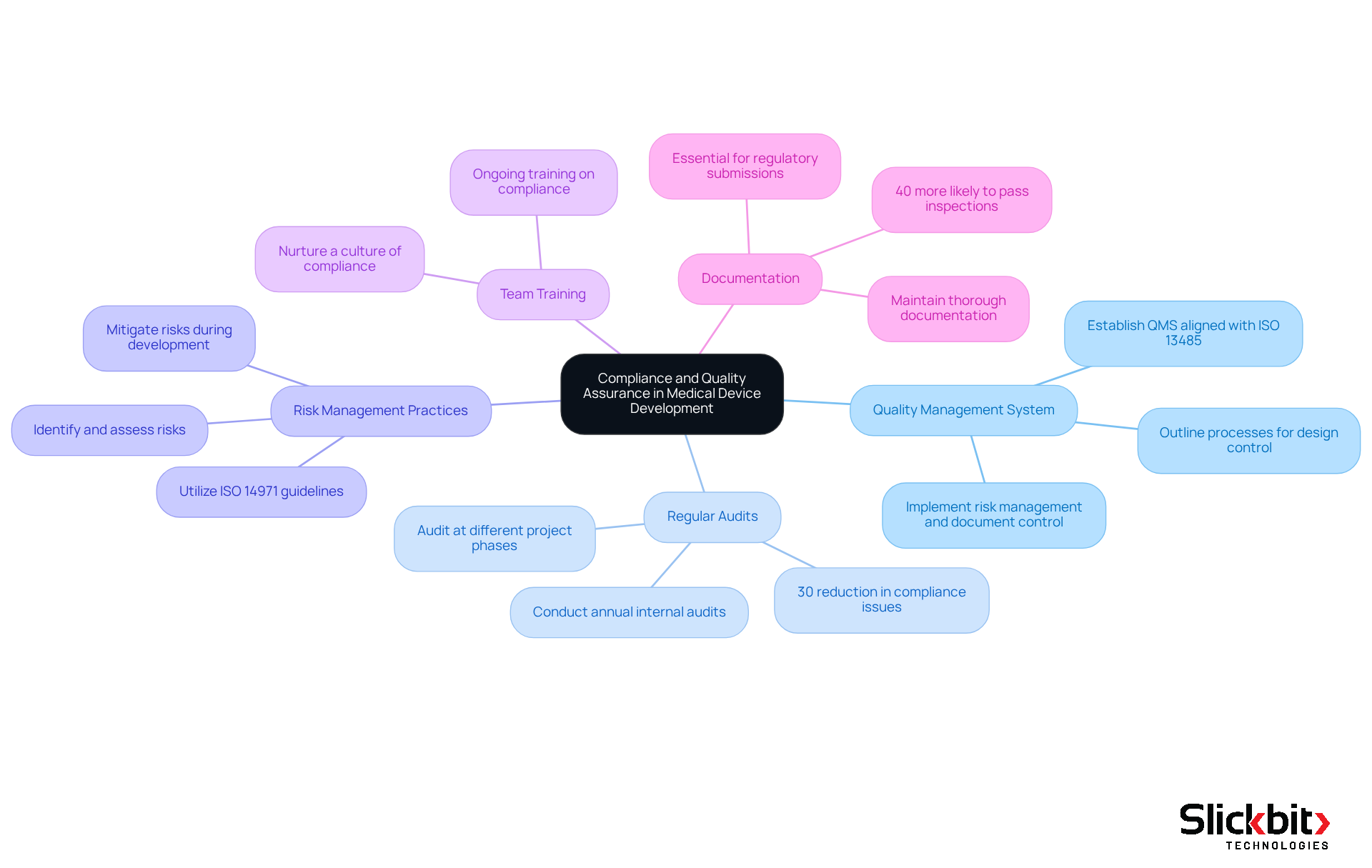
To ensure compliance and quality assurance throughout the medical device development, R&D managers must implement effective strategies.
-
Develop a Quality Management System (QMS): Establish a robust QMS that aligns with ISO 13485 standards. This system should outline processes for design control, risk management, and document control, ensuring a structured approach to quality.
-
Conduct Regular Audits: Schedule internal audits at least annually to assess compliance with established procedures and identify areas for improvement. Statistics indicate that companies conducting regular audits experience a 30% reduction in compliance-related issues. Furthermore, audits should be carried out at different phases of progress to ensure adherence to quality standards.
-
Implement Risk Management Practices: Utilize ISO 14971 guidelines to identify, assess, and mitigate risks associated with the device. A proactive strategy for risk management will aid in avoiding problems during creation and post-market use, consequently enhancing overall product safety.
-
Train Team Members: Provide ongoing training for team members on compliance requirements and quality assurance practices. This guarantees that all participants in the creation process comprehend their duties and the significance of upholding quality standards, nurturing a culture of compliance.
-
Document Everything: Maintain thorough documentation of all processes, decisions, and changes made during the project. This documentation is essential for regulatory submissions and for demonstrating compliance during audits. Case studies show that companies with comprehensive documentation practices are 40% more likely to pass regulatory inspections without issues.
By integrating compliance and quality assurance into the medical device development process, R&D leaders can effectively reduce risks and improve the chances of successful product launches.
Identify and Overcome Challenges in Medical Device Development
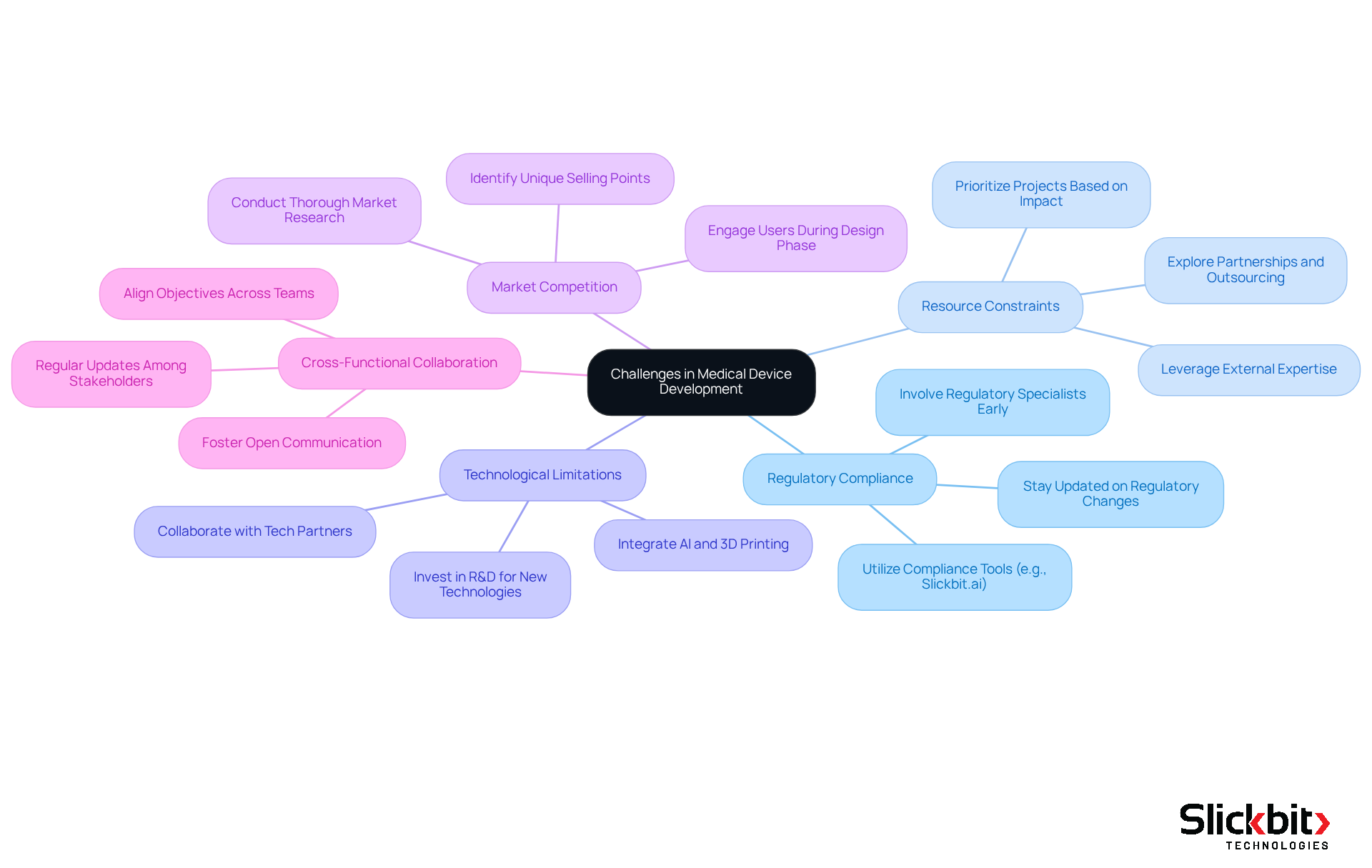
R&D managers encounter several common challenges in the field of medical device development and must implement effective strategies to overcome them.
-
Regulatory Compliance: The regulatory landscape is becoming increasingly complex, especially with updates anticipated in 2025. It is crucial to involve regulatory specialists early in the creation process to ensure that all compliance requirements are recognized and fulfilled. For example, manufacturers of Class D devices must apply for an IVDR conformity assessment by May 26, underscoring the urgency of proactive compliance measures. Tools like Slickbit.ai's Trend 483 can assist in identifying trends in systemic risks and repeat violations from FDA 483s, enabling users to search, filter, and access full 483s for deeper insights that enhance compliance efforts.
-
Resource Constraints: Limited budgets and personnel can significantly impede progress. R&D managers should prioritize projects based on their potential impact and explore partnerships or outsourcing options to supplement internal resources. Case studies indicate that companies leveraging external expertise can enhance their growth capabilities while managing costs effectively.
-
Technological Limitations: The demand for innovative devices often necessitates advanced technologies that may not be readily available. Investing in research and development to explore new technologies, alongside collaboration with tech partners, can provide access to cutting-edge solutions. For instance, the integration of AI and 3D printing is revolutionizing the personalization of medical tools, facilitating quicker prototyping and manufacturing. Moreover, AI systems can streamline operational processes, such as managing reservations and schedules, which can be advantageous in related operational contexts.
-
Market Competition: The medical equipment market is intensely competitive, requiring differentiation of products. Conducting thorough market research and engaging potential users during the design phase can help identify unique selling points. With the U.S. medical device market projected to grow at a CAGR of 6.99%, understanding market dynamics is vital for success.
-
Cross-Functional Collaboration: Effective cooperation among diverse groups is essential for success. R&D leaders should foster a culture of open communication and regular updates to ensure that all stakeholders are aligned and working towards shared objectives. This approach not only enhances group cohesion but also improves the overall effectiveness of the production process.
By proactively identifying and addressing these challenges, R&D leaders can significantly enhance their prospects for successful medical device development, ultimately leading to improved patient outcomes and market success.
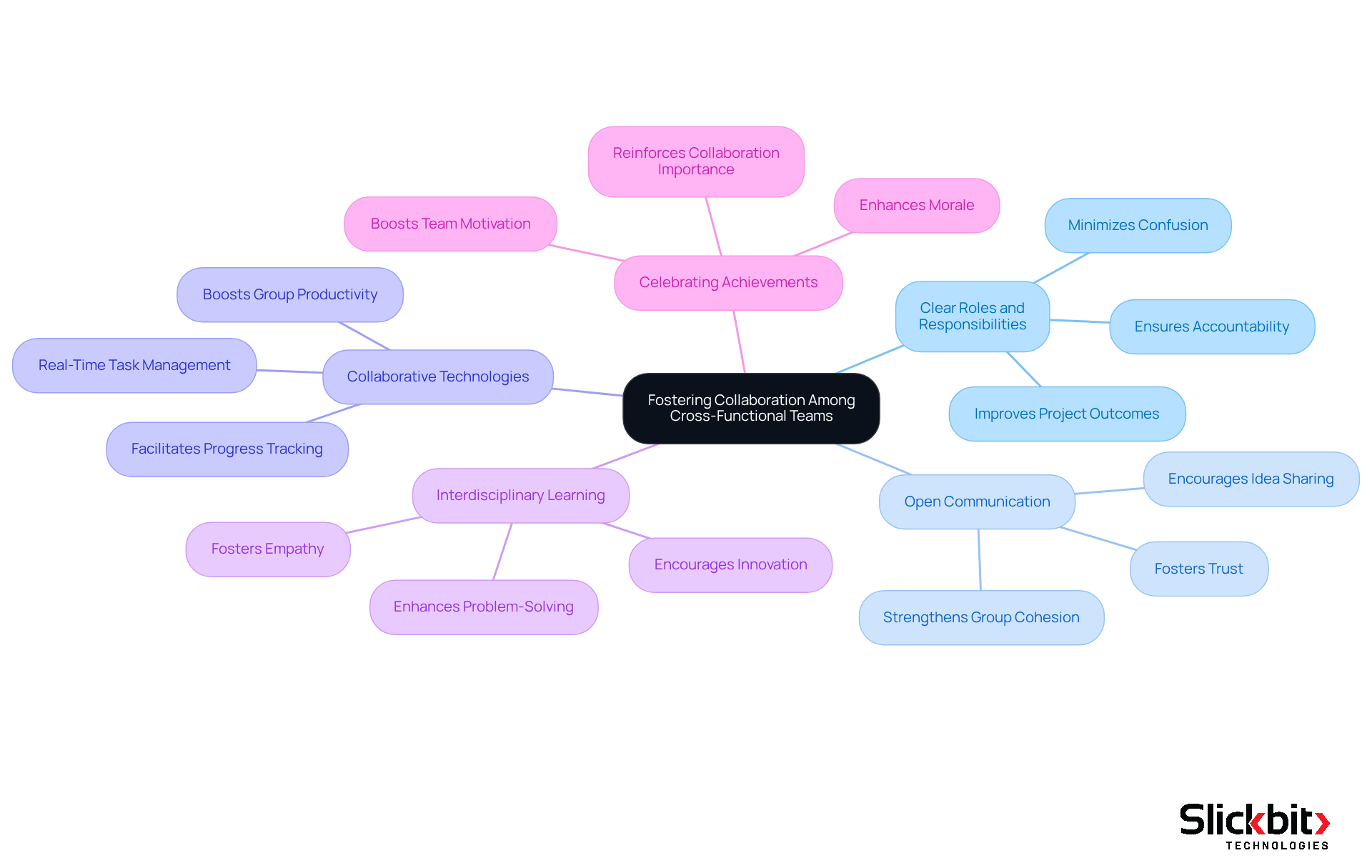
Foster Collaboration Among Cross-Functional Teams
To enhance collaboration among cross-functional teams, R&D managers must adopt effective strategies that drive innovation and improve efficiency in medical device development.
Establishing clear roles and responsibilities is crucial for minimizing confusion and ensuring accountability. By clearly defining each member's responsibilities, communication and decision-making are streamlined, leading to improved project outcomes. Research indicates that groups with well-defined roles are 1.9 times more likely to achieve above-median financial performance.
Furthermore, encouraging open communication is essential. Creating an environment where team members feel comfortable sharing ideas and feedback fosters collaboration. Regular meetings and collaborative tools promote open dialogue, strengthening group cohesion and trust. As Caitlin MacGregor suggests, understanding individuals' strengths and roles can significantly enhance collaborative efforts.
In addition, utilizing collaborative technologies is vital. Implementing project management and collaboration tools such as Slack, Trello, or Asana keeps teams connected and informed. These tools facilitate real-time task management and progress tracking, which are critical for maintaining momentum in fast-paced development environments. Investing in collaboration tools is essential for boosting group productivity, as highlighted in recent studies.
Promoting interdisciplinary learning also plays a key role. Encouraging team members to learn about each other's fields fosters empathy and enhances collaboration. By understanding the challenges faced by colleagues from diverse backgrounds, teams can engage in more effective problem-solving and innovation.
Finally, celebrating group achievements is necessary for enhancing morale and reinforcing the importance of collaboration in achieving project objectives. Acknowledging successes can significantly boost team motivation and commitment.
By implementing these strategies, R&D managers can cultivate a collaborative culture that not only drives innovation but also enhances the efficiency of medical device development.
Conclusion
Navigating the complex landscape of medical device development necessitates that R&D managers adopt a comprehensive approach, one that encompasses market trends, regulatory frameworks, technological innovations, and competitive analysis. Understanding these critical components allows managers to establish a robust foundation for successful product development, ensuring alignment with industry standards and responsiveness to market needs.
The article elucidates the essential phases of medical device development, spanning from concept and feasibility to post-market surveillance. Each stage requires meticulous attention to detail and an unwavering commitment to compliance and quality assurance. By implementing effective strategies—such as developing a robust Quality Management System, conducting regular audits, and fostering cross-functional collaboration—R&D leaders can significantly enhance their teams' productivity and innovation capabilities, ultimately facilitating successful product launches.
In conclusion, the importance of proactive strategies in overcoming challenges within the medical device sector is paramount. By prioritizing collaboration, comprehending regulatory requirements, and leveraging technological advancements, R&D managers not only navigate the complexities of device development but also contribute to improved patient outcomes and market success. Embracing these practices equips teams to meet the demands of a rapidly evolving industry, positioning them for long-term success in the competitive medical device market.
Frequently Asked Questions
What are the critical areas R&D managers must prioritize in medical device development?
R&D managers should prioritize market trends, regulatory frameworks, technological innovations, and the competitive landscape to effectively navigate the medical device development landscape.
Why is it important to analyze market trends in medical device development?
Analyzing market trends is essential because it helps identify the growing integration of AI in diagnostics and the rising demand for telehealth solutions, which reflect significant shifts towards technology-driven healthcare.
What role do regulatory frameworks play in medical device development?
Familiarity with regulatory frameworks, including FDA guidelines and ISO standards, is crucial for ensuring compliance throughout the development process and avoiding costly delays.
What are some key technological innovations in the medical device field?
Key technological innovations include advanced materials, IoT integration, and AI applications, such as AI-powered systems used in hospitals for analyzing medical images to improve diagnostic accuracy.
How can R&D managers understand the competitive landscape of the medical device industry?
R&D managers can understand the competitive landscape by identifying key players, analyzing their product offerings, strengths, and weaknesses, which will guide strategy and product positioning.
What are the main phases of the medical device development process?
The main phases include Concept and Feasibility, Design and Development, Verification and Validation, Regulatory Submission, and Post-Market Surveillance.
What happens during the Concept and Feasibility phase?
This phase involves brainstorming innovative ideas, conducting thorough market research, and evaluating the practicality of the proposed device, with active engagement from stakeholders for feedback.
What is the significance of the Design and Development phase?
This phase focuses on crafting detailed design specifications and developing prototypes while documenting design decisions to ensure compliance with regulatory standards.
What is involved in the Verification and Validation phase?
This phase includes rigorous testing to verify that the device meets design specifications and validation to confirm that it fulfills its intended use, requiring a comprehensive testing plan.
What is the purpose of the Regulatory Submission phase?
The purpose is to prepare and submit necessary documentation to regulatory bodies for approval, compiling data from the verification and validation phases to demonstrate the device's safety and efficacy.
What is the importance of Post-Market Surveillance?
Post-Market Surveillance is essential for ongoing monitoring to ensure continued compliance and to gather feedback for future enhancements after the product launch.




