Overview
Mean residence time (MRT) stands as a pivotal pharmacokinetic parameter, signifying the average duration a drug remains within the body. This metric profoundly influences both drug efficacy and safety throughout pharmaceutical development. Accurately estimating MRT is paramount, as it informs dosing regimens and ensures regulatory compliance. Furthermore, understanding the factors that impact MRT is essential for optimizing therapeutic outcomes. By grasping these concepts, professionals can enhance their approach to drug development and patient care.
Introduction
Understanding the nuances of Mean Residence Time (MRT) is crucial for pharmaceutical R&D managers navigating the complexities of drug development. This vital pharmacokinetic parameter not only informs dosing regimens and medication efficacy but also plays a significant role in regulatory compliance and patient safety.
However, with various factors influencing MRT—from drug formulation to individual patient characteristics—how can R&D teams ensure accurate predictions and optimize therapeutic outcomes?
This article delves into the intricacies of MRT, offering key insights and methodologies that empower pharmaceutical professionals to enhance their drug development strategies.
Define Mean Residence Time and Its Importance in Pharmaceuticals
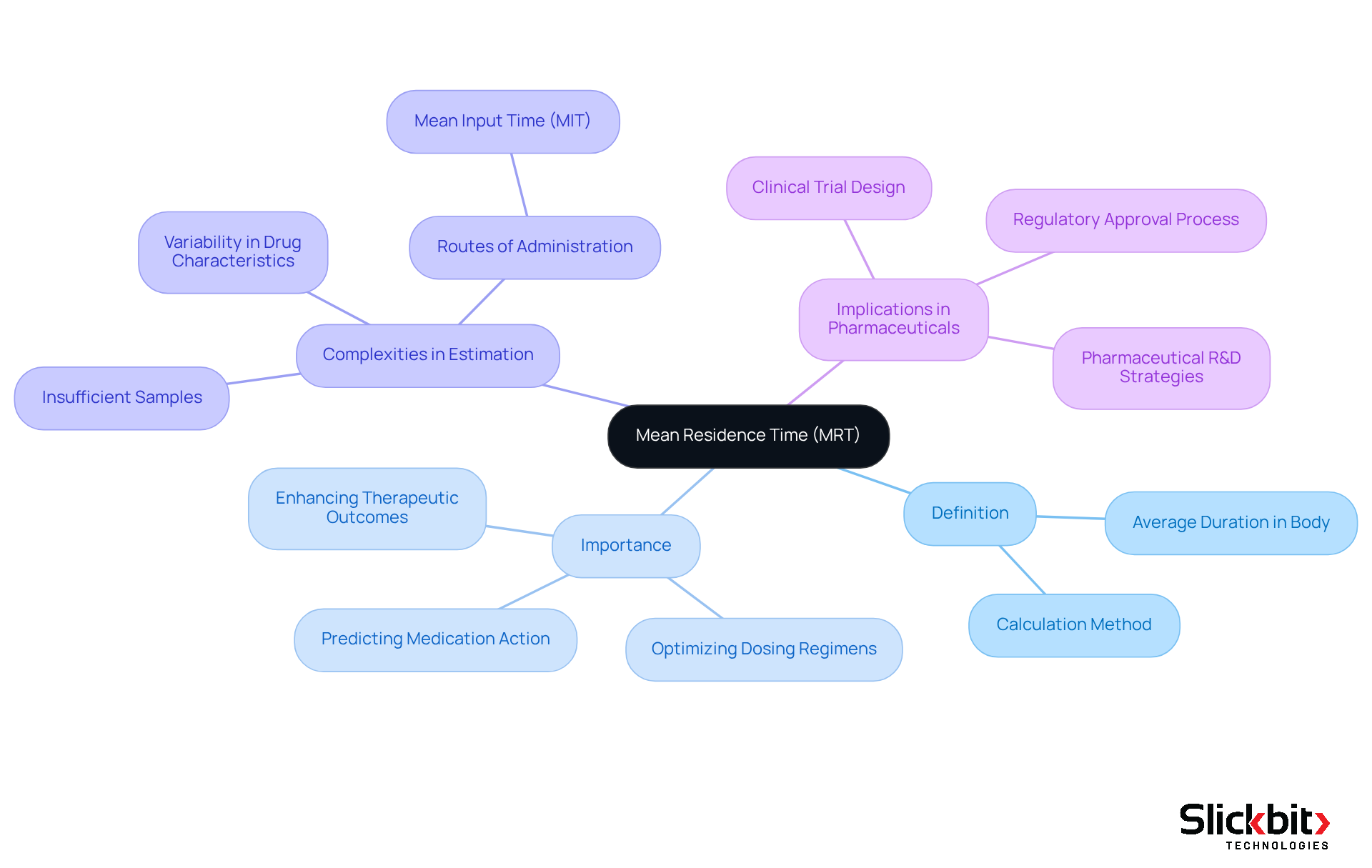
Mean residence time (MRT) is an essential pharmacokinetic parameter that reflects the average duration a substance stays in the body after administration. It is determined by dividing the total time a substance is present in the system by the number of molecules administered. Understanding mean residence time (MRT) is vital in pharmaceuticals, as it helps predict the duration of medication action, optimize dosing regimens, and clarify the pharmacokinetic profile of substances. A precisely defined mean residence time (MRT) can significantly enhance therapeutic outcomes and minimize side effects, making it an essential consideration in medication development.
Recent studies underscore the complexities involved in accurately estimating MRT, particularly when there are insufficient samples to ascertain the terminal elimination rate constant. This highlights the necessity for meticulous sampling and analysis in pharmacokinetic studies to ensure reliable estimates of mean residence time. For example, in a thought experiment involving ten chemical molecules, the calculated MRT was found to be 85.6 minutes, illustrating the variability that can arise based on individual compound characteristics.
Furthermore, the significance of mean residence time transcends mere calculations; it plays a pivotal role in the design of clinical trials and in the regulatory approval process. By providing insights into medication behavior within the body, the mean residence time (MRT) informs decisions that can lead to more effective and safer therapeutic options. Consequently, pharmaceutical R&D managers must prioritize mean residence time in their strategies to align with regulatory expectations and enhance patient outcomes.
Explore Factors Affecting Mean Residence Time in Drug Development
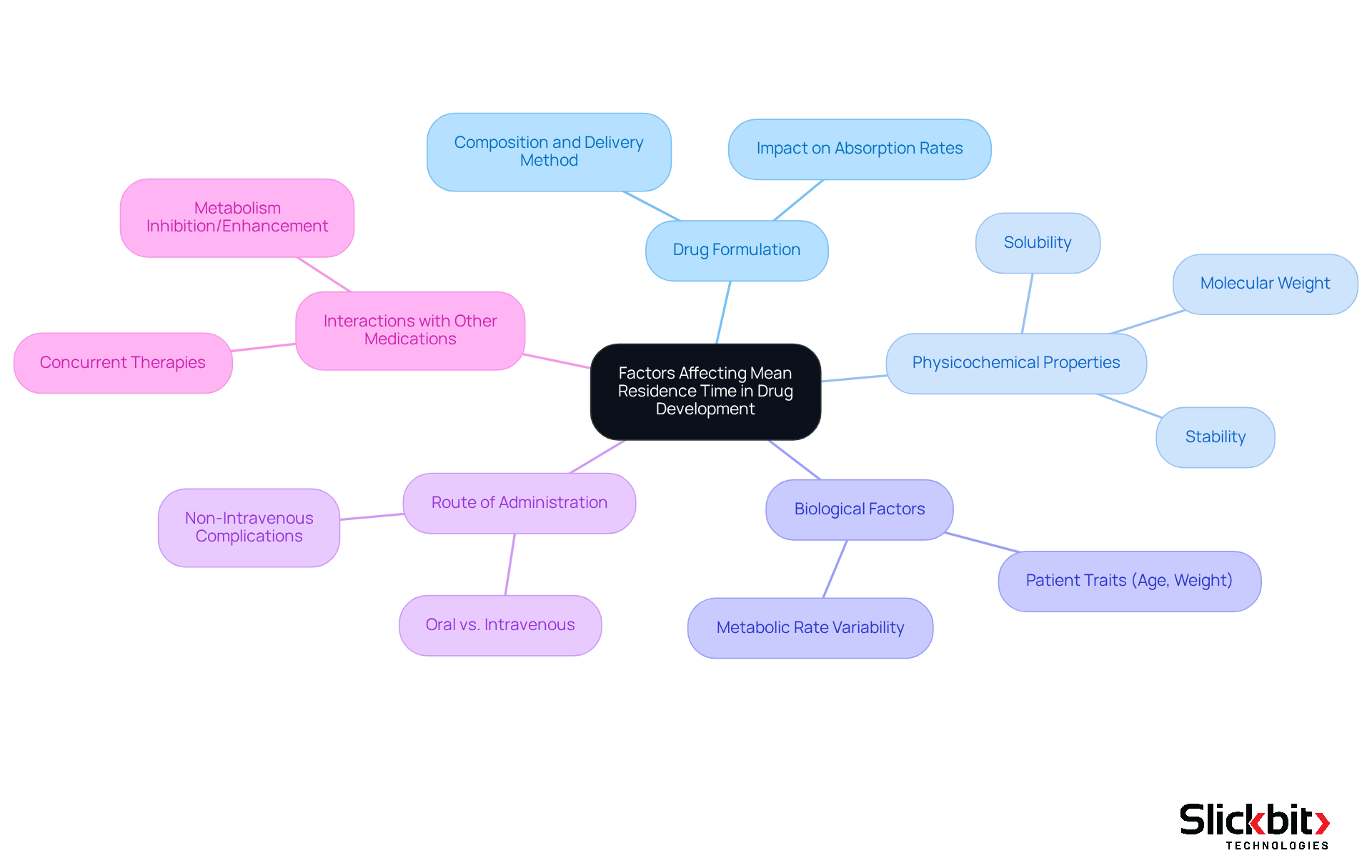
The mean residence time (MRT) in drug development is influenced by several critical factors.
-
Drug Formulation: The specific composition and delivery method of a drug play a pivotal role in determining its absorption and elimination rates. The formulation can dictate how rapidly a medication enters systemic circulation, thus impacting its mean residence time.
-
Physicochemical Properties: Key characteristics such as solubility, stability, and molecular weight significantly impact the duration a substance remains in circulation. Drugs with higher solubility often exhibit improved absorption rates, which can lead to a shorter MRT if eliminated rapidly.
-
Biological Factors: Individual patient traits, including age, weight, and metabolic rate, can influence medication metabolism and clearance. For instance, older patients may process medications differently, potentially prolonging the mean residence time compared to younger groups.
-
Route of Administration: The method of substance delivery—whether oral, intravenous, or otherwise—can lead to substantial variations in MRT. Non-intravenous routes often require consideration of mean residence time (MRT), complicating calculations and necessitating tailored approaches based on administration methods.
-
Interactions with Other Medications: Concurrent therapies can significantly influence the pharmacokinetics of a substance, thereby altering its MRT. Certain substances may inhibit or enhance the metabolism of others, resulting in unforeseen alterations in mean residence time.
Comprehending these elements allows R&D teams to forecast and influence the mean residence time more effectively, ultimately improving treatment efficacy and safety. Recent studies have indicated that optimizing formulation can result in enhanced mean residence time (MRT) outcomes, which is vital for the success of clinical trials and the overall therapeutic creation process. Notably, the success rate of clinical trials rose by 11.3 percentage points when non-industrial partners participated, underscoring the importance of collaboration in medicine development. Furthermore, delays in clinical trials can lead to a loss of $8 million in revenue per day for pharmaceutical discovery firms, highlighting the financial consequences of optimizing mean residence time. A practical example can be seen in the case study 'Calculating mean residence time from Molecule Data,' where the mean residence time was determined to be 85.6 minutes, illustrating how empirical data can be utilized to compute this pharmacokinetic parameter.
Calculate Mean Residence Time: Methods and Best Practices
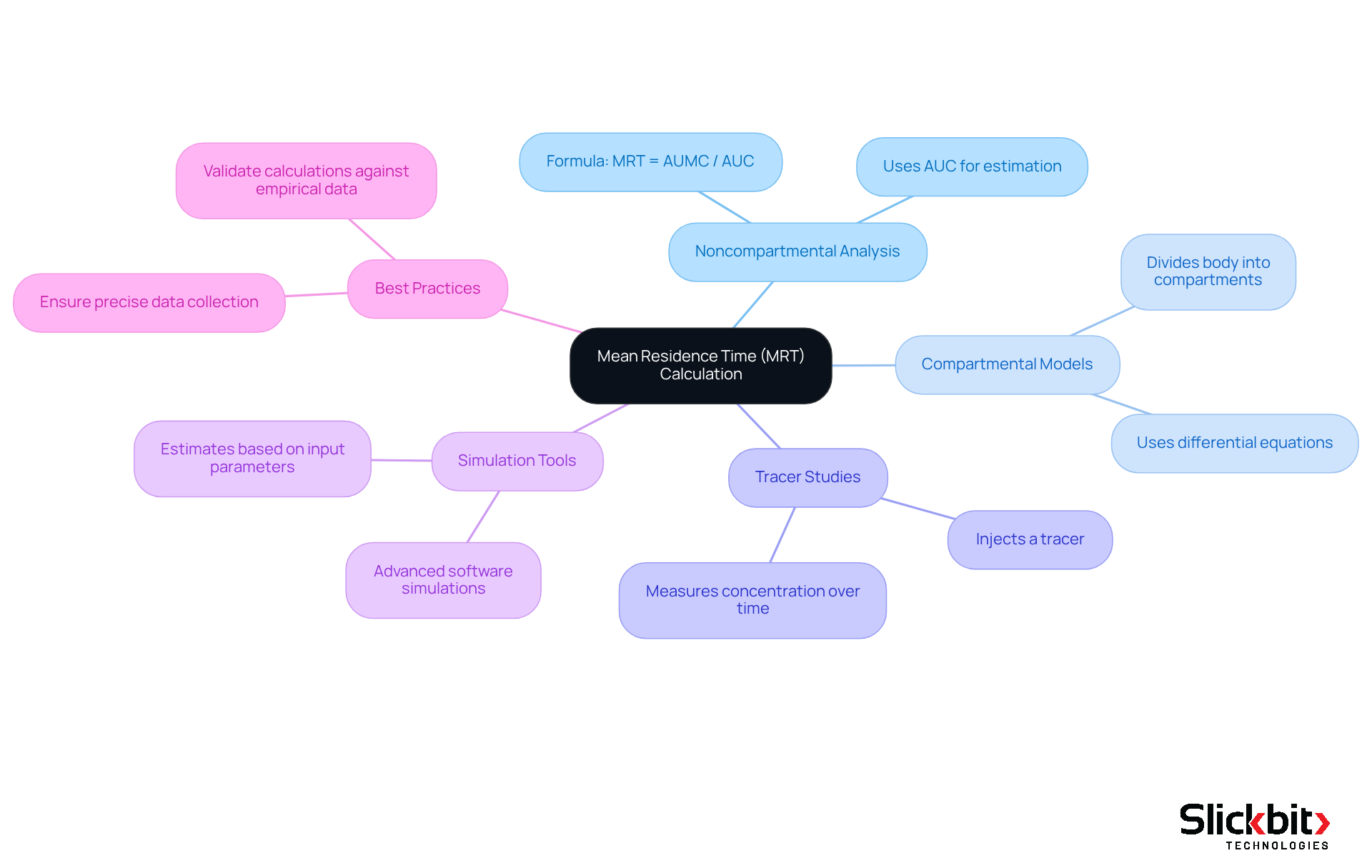
Calculating Mean Residence Time (MRT) is essential in pharmacokinetics, and it can be accomplished through various methods:
-
Noncompartmental Analysis: This method employs the area under the concentration-time curve (AUC) to estimate MRT. The formula is:
MRT = AUMC / AUCwhere AUMC represents the area under the first moment curve.
-
Compartmental Models: These models divide the body into compartments, utilizing differential equations to describe the distribution and elimination of substances. The parameters of the model can be used to derive the mean residence time.
-
Tracer Studies: By injecting a tracer and measuring its concentration over time, researchers can calculate the mean residence time based on the resulting data.
-
Simulation Tools: Advanced software can simulate the behavior of substances within the body, providing estimates of mean residence time based on various input parameters.
Best practices include ensuring precise data collection, employing appropriate models for the substance's pharmacokinetics, and validating calculations against empirical data. This ensures the reliability of the estimates of mean residence time, ultimately enhancing the understanding of drug behavior in the body.
Analyze the Impact of Mean Residence Time on Drug Efficacy and Safety
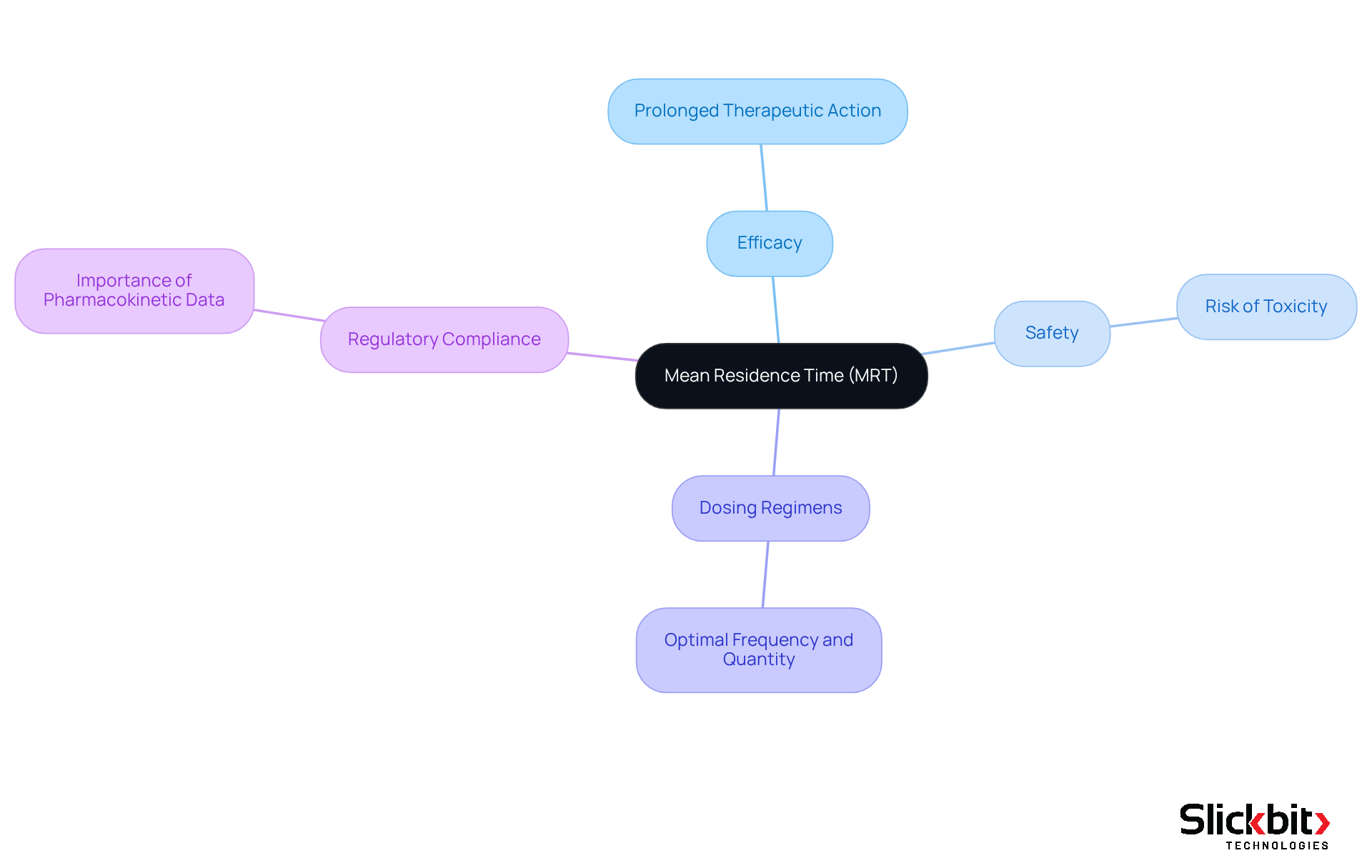
Mean residence time (MRT) plays a significant role in determining medication effectiveness and safety, making it a crucial factor in pharmaceutical development. Understanding mean residence time is essential for addressing several key considerations in this field.
-
Efficacy: An extended MRT can enhance drug efficacy by maintaining therapeutic levels at the target site. This is particularly vital for drugs that rely on consistent plasma concentrations for optimal performance. For example, a longer MRT can ensure sustained medication levels, facilitating prolonged therapeutic action, especially for substances that require stable plasma concentrations to be effective.
-
Safety: Conversely, an excessively prolonged MRT may lead to toxicity, as sustained exposure to a substance increases the risk of adverse effects. Thus, achieving the right balance between effectiveness and safety is crucial in the medication development process. Recent findings highlight that prolonged exposure can result in heightened adverse effects, underscoring the importance of careful monitoring of the mean residence time.
A comprehensive understanding of mean residence time is vital for designing effective dosing regimens. It aids in determining the frequency and dosage necessary to maintain therapeutic levels while minimizing toxicity risks. By understanding the mean residence time (MRT), researchers can establish the optimal frequency and quantity of medication administration required to sustain therapeutic levels without inducing toxicity.
- Regulatory Compliance: Regulatory bodies often mandate extensive pharmacokinetic data, including mean residence time (MRT), to evaluate the safety and effectiveness of new pharmaceuticals. A solid understanding of mean residence time can streamline regulatory submissions and enhance approval processes. For instance, regulatory authorities frequently require comprehensive pharmacokinetic information, including MRT, to assess the safety and effectiveness of new medications. A thorough grasp of MRT can facilitate smoother regulatory submissions and approvals.
By thoroughly examining the implications of mean residence time on medication performance, R&D teams can make informed decisions that enhance both the effectiveness and safety of their products. Incorporating case studies and expert opinions on MRT can further deepen the understanding of its impact on drug safety and efficacy.
Conclusion
Understanding mean residence time (MRT) is crucial for pharmaceutical R&D managers, as it directly influences drug efficacy, safety, and overall therapeutic outcomes. By grasping the nuances of MRT—its definition and significance—professionals can optimize drug development processes and ensure compliance with regulatory standards. Prioritizing MRT in R&D strategies not only aligns with regulatory expectations but also enhances patient care through improved medication management.
The article delves into various factors affecting mean residence time, including:
- Drug formulation
- Physicochemical properties
- Biological factors
- Route of administration
- Medication interactions
Each of these elements plays a vital role in determining how long a drug remains active in the body, impacting dosing regimens and therapeutic effectiveness. Furthermore, the article outlines practical methods for calculating MRT, emphasizing best practices that ensure accurate and reliable pharmacokinetic data.
In conclusion, a comprehensive understanding of mean residence time is essential for advancing drug development and optimizing patient outcomes. R&D managers are encouraged to integrate MRT considerations into their workflows, fostering collaboration and innovation in pharmaceutical research. By doing so, they can contribute to the creation of safer, more effective medications that meet the evolving needs of patients and healthcare providers alike.
Frequently Asked Questions
What is mean residence time (MRT) in pharmaceuticals?
Mean residence time (MRT) is a pharmacokinetic parameter that indicates the average duration a substance remains in the body after administration. It is calculated by dividing the total time a substance is present in the system by the number of molecules administered.
Why is mean residence time (MRT) important in pharmaceuticals?
MRT is crucial because it helps predict how long a medication will act, optimizes dosing regimens, and clarifies the pharmacokinetic profile of substances. A well-defined MRT can enhance therapeutic outcomes and reduce side effects.
What challenges are associated with estimating mean residence time (MRT)?
Accurately estimating MRT can be complex, especially when there are insufficient samples to determine the terminal elimination rate constant. This emphasizes the need for careful sampling and analysis in pharmacokinetic studies.
Can you provide an example of mean residence time (MRT) calculation?
In a thought experiment involving ten chemical molecules, the calculated MRT was found to be 85.6 minutes, demonstrating the variability that can occur based on the characteristics of individual compounds.
How does mean residence time (MRT) influence clinical trials and regulatory approval?
MRT provides insights into how medications behave in the body, which is essential for designing clinical trials and for the regulatory approval process. It informs decisions that can lead to more effective and safer therapeutic options.
What should pharmaceutical R&D managers consider regarding mean residence time (MRT)?
Pharmaceutical R&D managers should prioritize mean residence time in their strategies to meet regulatory expectations and improve patient outcomes.




