Overview
The article delineates a systematic approach for R&D managers to proficiently execute GMP audits, underscoring the critical nature of preparation, precise scope definition, and comprehensive documentation. It elaborates on each phase of the audit process, including:
- The engagement of staff for valuable insights
- The navigation of common challenges
These elements collectively fortify compliance with regulatory standards and amplify product safety within the pharmaceutical sector.
Introduction
A Good Manufacturing Practice (GMP) audit is not merely a regulatory formality; it serves as a crucial process that safeguards product quality and patient safety within the pharmaceutical industry. For R&D managers, mastering the intricacies of GMP audits presents an invaluable opportunity to enhance operational efficiency and ensure compliance with stringent standards set by authorities such as the FDA and EMA. However, the journey toward a successful audit is fraught with challenges, ranging from documentation issues to staff resistance. Consequently, how can R&D leaders adeptly navigate these complexities to not only meet compliance but also drive continuous improvement within their organizations?
Define GMP Audit and Its Importance
A Good Manufacturing Practice (GMP) audit serves as a comprehensive evaluation of a facility's operations, processes, and quality systems, aimed at ensuring adherence to regulatory standards. These evaluations, such as the GMP audit, are crucial in the pharmaceutical industry, as they not only identify areas for enhancement but also ensure product quality and compliance with regulations established by entities like the FDA and EMA. Recent findings from FDA inspections have underscored the necessity of rigorous GMP practices, revealing significant deficiencies that can jeopardize product safety and efficacy. For instance, a recent FDA Warning Letter highlighted serious GMP violations at a manufacturing facility, emphasizing the critical need for thorough compliance checks.
By conducting a GMP audit regularly, R&D managers can effectively mitigate risks associated with non-compliance, improve operational efficiency, and guarantee the safety and efficacy of pharmaceutical products. These evaluations represent a proactive approach to detect potential issues before they escalate, thereby protecting patient health and preserving confidence in pharmaceutical products. Furthermore, engaging third-party auditors can provide an unbiased perspective and expert recommendations, facilitating continuous improvement in quality systems.
Integrating AI technologies, such as those provided by Trend 483, can further enhance the GMP examination by identifying patterns in systemic risks and recurring violations from FDA 483s. This integration of AI insights empowers R&D managers to make informed choices and bolster regulatory efforts, ultimately supporting the successful commercialization of safe and effective pharmaceutical products.
Understanding the importance of GMP audits is essential for mastering the assessment process, as it reinforces the necessity for strict adherence to quality standards throughout the drug development lifecycle. The benefits of performing GMP evaluations extend beyond mere compliance; they also contribute to enhancing processes, reducing costs, and ultimately facilitating the successful marketing of safe and effective pharmaceutical products.
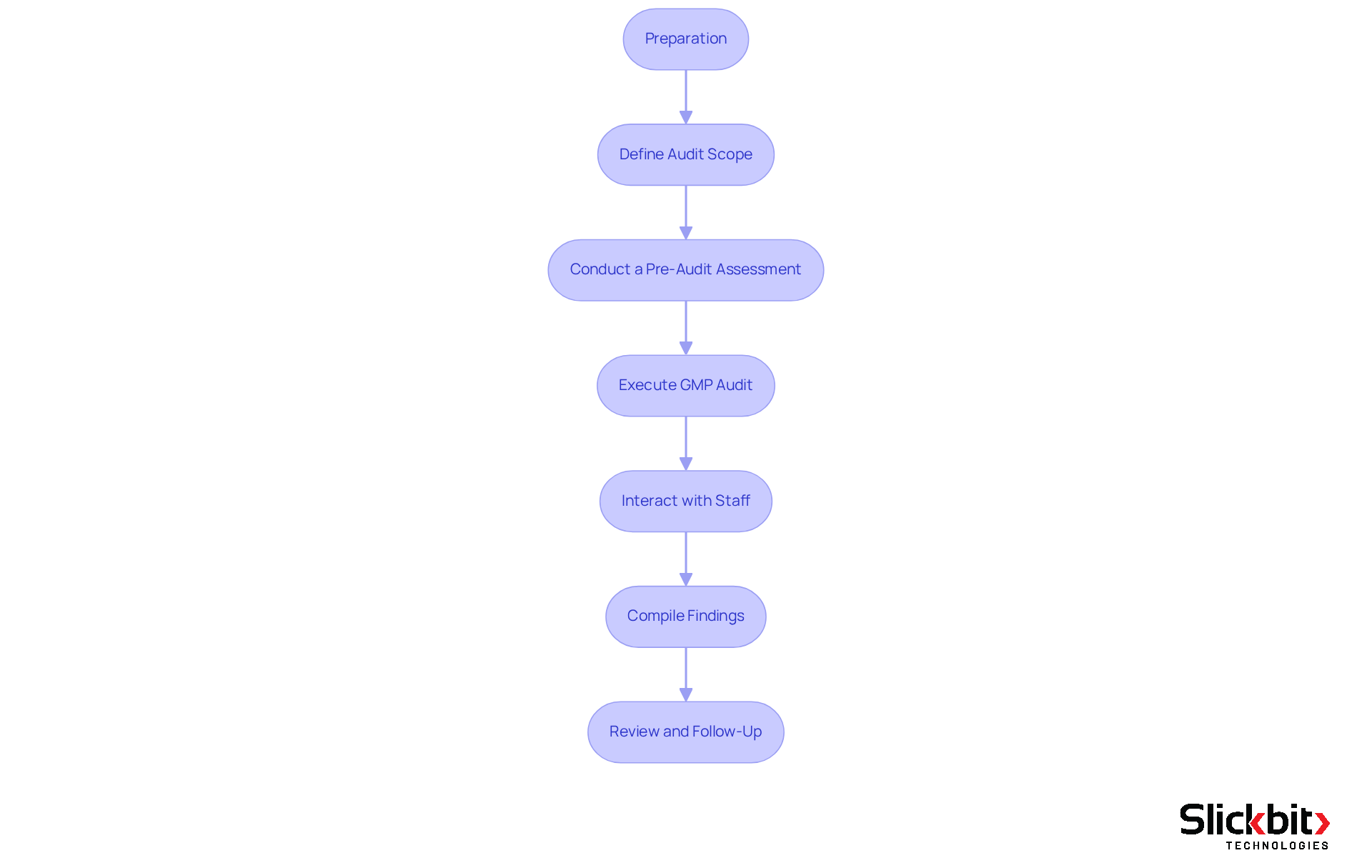
Step-by-Step Process for Conducting a GMP Audit
-
Preparation: Begin by gathering all necessary documentation, including Standard Operating Procedures (SOPs), batch records, and prior inspection reports. It is crucial that all team members involved in the evaluation understand their roles and responsibilities, as this clarity is essential for a seamless operation.
-
Define Audit Scope: Clearly delineate the audit's scope, specifying which procedures, departments, or facilities will be reviewed. This focused approach ensures that all critical areas are addressed, thereby minimizing the risk of oversight.
-
Conduct a Pre-Audit Assessment: Perform a preliminary review of current methods and systems. This may include informal interviews with staff and a walkthrough of the facility to identify potential areas of concern. Such proactive measures can significantly enhance readiness.
-
Execute the GMP audit by utilizing a structured checklist to evaluate compliance with GMP standards. Document findings meticulously, noting any deviations from established practices. Audits typically last between one to five days, depending on the facility's size and complexity, making thorough documentation essential.
-
Interact with Staff: Throughout the review, actively engage with staff to gain insights into their processes and identify potential enhancements. This collaborative approach can uncover valuable information that may not be captured through documentation alone.
-
Compile Findings: After the review, consolidate all findings into a comprehensive report. Highlight areas of non-compliance, potential risks, and recommendations for corrective actions. This report serves as a critical tool for continuous improvement in the context of a GMP audit.
-
Review and Follow-Up: Present the assessment findings to relevant stakeholders and create an action plan to address identified issues. Arrange subsequent evaluations to confirm that corrective measures have been efficiently executed, as continuous adherence is crucial for preserving product quality and regulatory standards. Incorporating best practices, such as conducting mock audits and utilizing audit management software, can streamline the preparation phase and enhance overall compliance readiness.
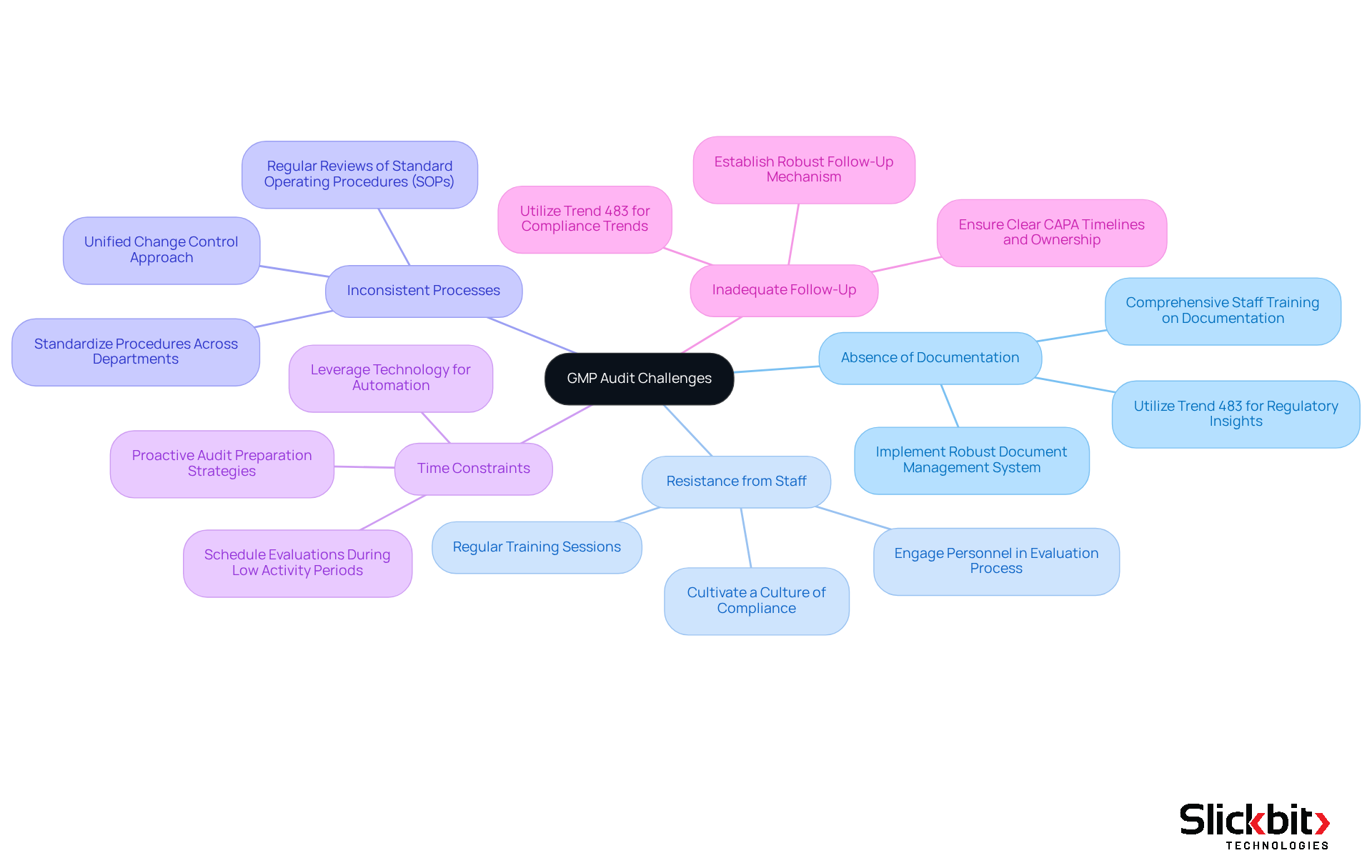
Troubleshoot Common GMP Audit Challenges
-
Absence of Documentation: Addressing documentation issues requires meticulous recording of all procedures and comprehensive training for staff on maintaining precise records. Implementing a robust document management system streamlines this process, thereby reducing the risk of non-conformities. Furthermore, employing Slickbit's Trend 483 significantly enhances documentation efforts by identifying regulatory patterns and providing deeper insights into FDA 483s, which is essential for preventing major regulatory failures.
-
Resistance from Staff: Cultivating a culture of compliance is paramount. Engaging personnel in the evaluation process emphasizes the advantages of a GMP audit, fostering a sense of ownership. Regular training sessions effectively address concerns and encourage cooperation, thereby mitigating resistance to the GMP audit. Data indicates that untrained employees substantially increase the likelihood of oversight errors, underscoring the necessity of effective training programs.
-
Inconsistent processes can hinder a GMP audit; therefore, standardizing procedures across departments minimizes variability and enhances compliance. Regular reviews and updates of Standard Operating Procedures (SOPs) ensure alignment with best practices, which is vital for maintaining consistency. Inconsistent change control has been linked to significant non-conformances, highlighting the importance of a unified approach.
-
Time Constraints: Scheduling evaluations during periods of reduced activity diminishes interference with operations. Leveraging technology, such as Trend 483, automates data collection and analysis, significantly conserving time and resources. This proactive approach alleviates the stress associated with GMP audit preparations, particularly during peak operational periods.
-
Inadequate Follow-Up: Establishing a robust follow-up mechanism is critical for tracking the implementation of corrective actions. Trend 483 provides valuable insights into compliance trends, ensuring that each Corrective and Preventive Action (CAPA) includes a clear timeline and ownership, along with evidence of effectiveness to demonstrate commitment to quality and compliance.
Conclusion
Conducting a GMP audit is a critical process that ensures compliance with regulatory standards in the pharmaceutical industry, safeguarding product quality and patient safety. Understanding the significance of these audits allows R&D managers to adopt a proactive stance towards compliance, ultimately enhancing operational efficiency and maintaining trust in pharmaceutical products.
This article outlines a comprehensive step-by-step process for executing a GMP audit, emphasizing the importance of preparation, clear scope definition, thorough documentation, and effective staff engagement. It addresses common challenges faced during audits, such as documentation issues and staff resistance, while providing actionable solutions to overcome these obstacles. Furthermore, the integration of technology, particularly AI tools like Trend 483, is highlighted as a means to streamline the audit process and improve compliance readiness.
Ultimately, mastering the GMP audit process transcends merely meeting regulatory requirements; it fosters a culture of quality and continuous improvement within organizations. R&D managers are encouraged to embrace these audits as opportunities for growth and excellence, ensuring that pharmaceutical products are not only compliant but also safe and effective for patients. Taking action now to implement these practices can lead to a more robust and reliable pharmaceutical development landscape.
Frequently Asked Questions
What is a GMP audit?
A Good Manufacturing Practice (GMP) audit is a comprehensive evaluation of a facility's operations, processes, and quality systems, aimed at ensuring adherence to regulatory standards in the pharmaceutical industry.
Why are GMP audits important?
GMP audits are crucial because they identify areas for enhancement, ensure product quality, and confirm compliance with regulations set by entities like the FDA and EMA. They help mitigate risks associated with non-compliance and protect patient health.
What can recent FDA inspections reveal about GMP practices?
Recent FDA inspections have highlighted significant deficiencies in GMP practices, which can jeopardize product safety and efficacy. For example, an FDA Warning Letter pointed out serious GMP violations at a manufacturing facility.
How can regular GMP audits benefit R&D managers?
Regular GMP audits enable R&D managers to mitigate risks, improve operational efficiency, and ensure the safety and efficacy of pharmaceutical products by detecting potential issues before they escalate.
What role do third-party auditors play in GMP audits?
Third-party auditors provide an unbiased perspective and expert recommendations, facilitating continuous improvement in quality systems during GMP audits.
How can AI technologies enhance GMP audits?
AI technologies, such as those offered by Trend 483, can identify patterns in systemic risks and recurring violations from FDA 483s, empowering R&D managers to make informed decisions and improve regulatory compliance.
What are the broader benefits of performing GMP evaluations?
Beyond compliance, GMP evaluations contribute to enhancing processes, reducing costs, and facilitating the successful marketing of safe and effective pharmaceutical products.




