Overview
This article serves as an authoritative guide for R&D managers aiming to master drug regulatory affairs. It outlines essential steps for ensuring compliance, managing clinical trials, and facilitating market approval for new drugs. Understanding regulatory frameworks is crucial; therefore, this article delves into how these frameworks can be navigated effectively.
Furthermore, the utilization of AI tools is emphasized as a means to enhance efficiency in the regulatory process. Maintaining proactive communication with regulatory authorities is also highlighted, as it significantly enhances the likelihood of successful drug submissions and approvals.
By following these guidelines, R&D managers can position themselves for success in the complex landscape of drug regulation.
Introduction
Mastering drug regulatory affairs is essential for research and development managers as they navigate the intricate landscape of pharmaceutical regulations. The complexities of compliance not only protect public health but also expedite the drug approval process, ultimately benefiting both organizations and patients alike. However, with regulations continuously evolving and advanced technologies being integrated, R&D leaders face the pressing challenge of ensuring compliance while simultaneously driving innovation.
How can they effectively achieve this balance?
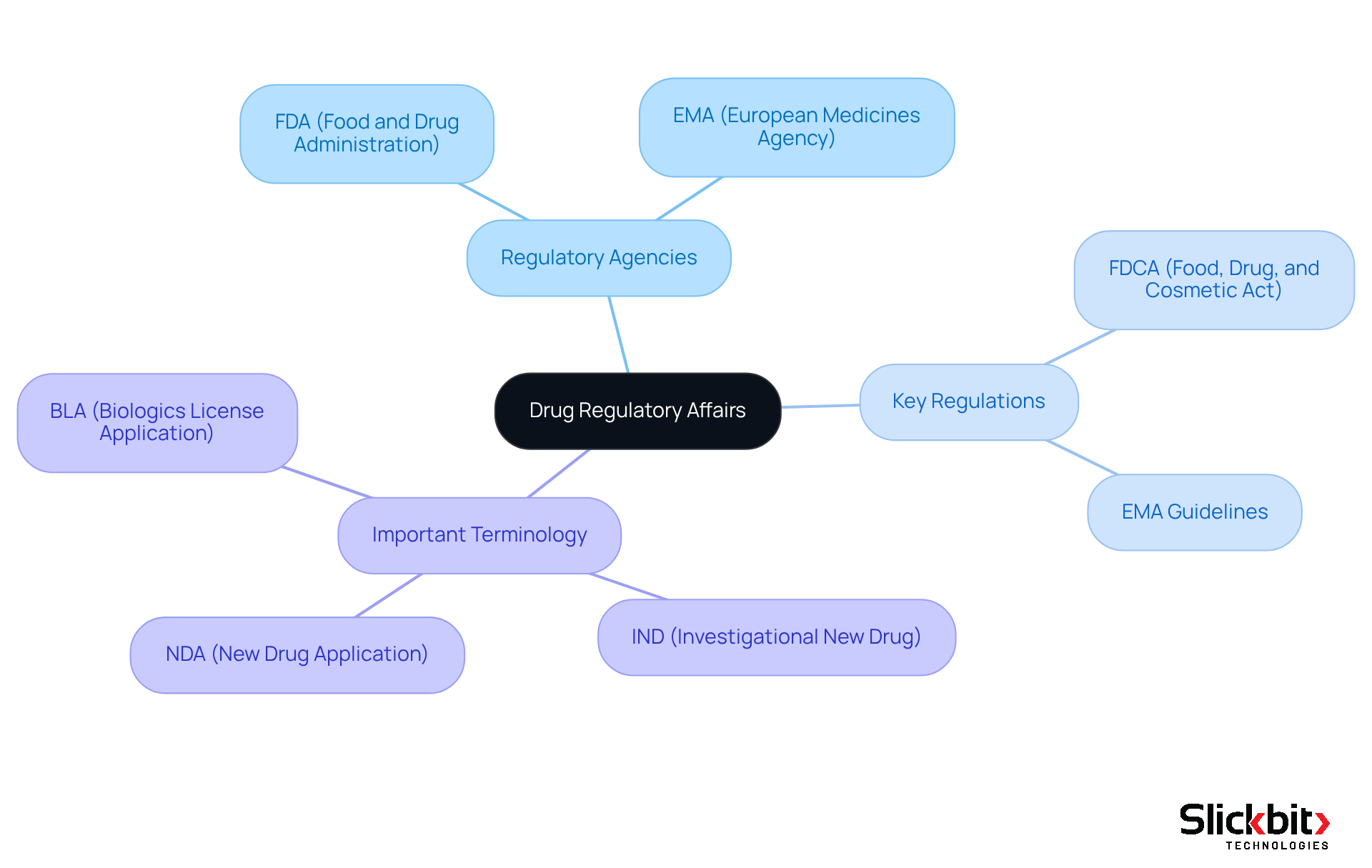
Understand the Basics of Drug Regulatory Affairs
To master drug regulatory affairs, it is essential to become familiar with key concepts that serve as the foundation of this field. First, understand the roles of regulatory agencies such as the FDA and EMA, which oversee the drug approval processes critical to ensuring public safety. Next, learn about key regulations, including the Food, Drug, and Cosmetic Act (FDCA) and the guidelines set forth by the European Medicines Agency (EMA). Additionally, familiarize yourself with important terminology, such as:
- IND (Investigational New Drug)
- NDA (New Drug Application)
- BLA (Biologics License Application)
By grasping these fundamentals, you will be better equipped to navigate the compliance environment effectively and improve your expertise in drug regulatory affairs.
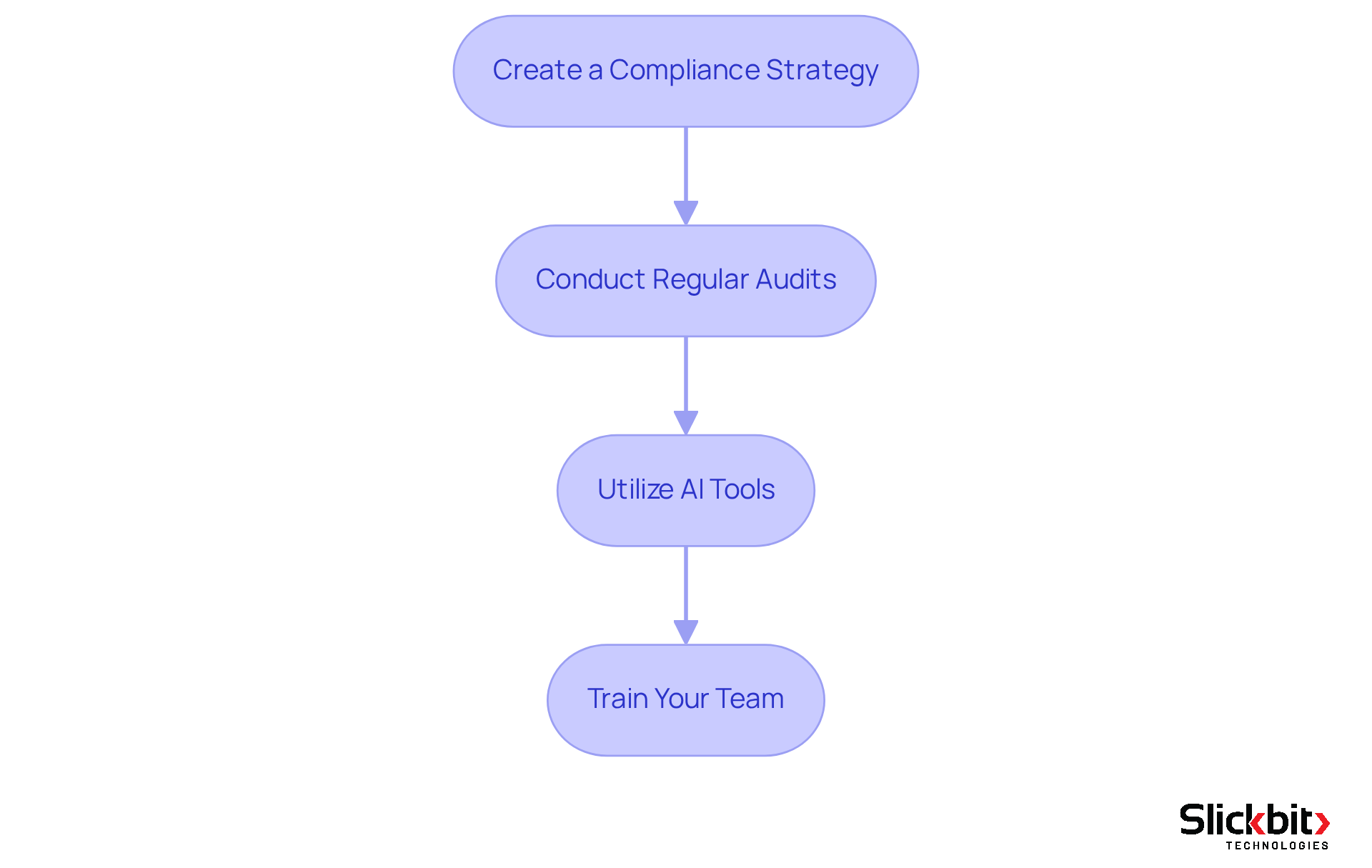
Ensure Compliance at Every Stage of Development
To ensure compliance throughout the drug development process, it is essential to consider the following steps:
- Create a Compliance Strategy: Design a comprehensive plan that outlines compliance needs for every stage of development, ensuring conformity with legal standards.
- Conduct Regular Audits: Implement a schedule for periodic audits to assess compliance with legal standards and internal policies. Regular audits are vital in drug regulatory affairs, as they help identify gaps and enhance operational efficiency, ultimately facilitating timely compliance submissions.
- Utilize AI Tools: Leverage AI-driven regulatory monitoring tools that can continuously evaluate workflows and flag potential issues in real-time. For instance, biopharma firms have effectively utilized AI solutions to uncover concealed risk factors, accelerating drug approval processes and preventing costly delays related to regulations.
- Train Your Team: Provide ongoing instruction for your team on legal obligations and best practices. This investment in knowledge ensures that your team is equipped to navigate the complexities of regulations effectively.
By incorporating adherence into your development plan, you can mitigate risks and enhance the likelihood of successful submissions. The integration of AI tools not only simplifies compliance monitoring but also positions your organization advantageously in a rapidly evolving oversight environment.
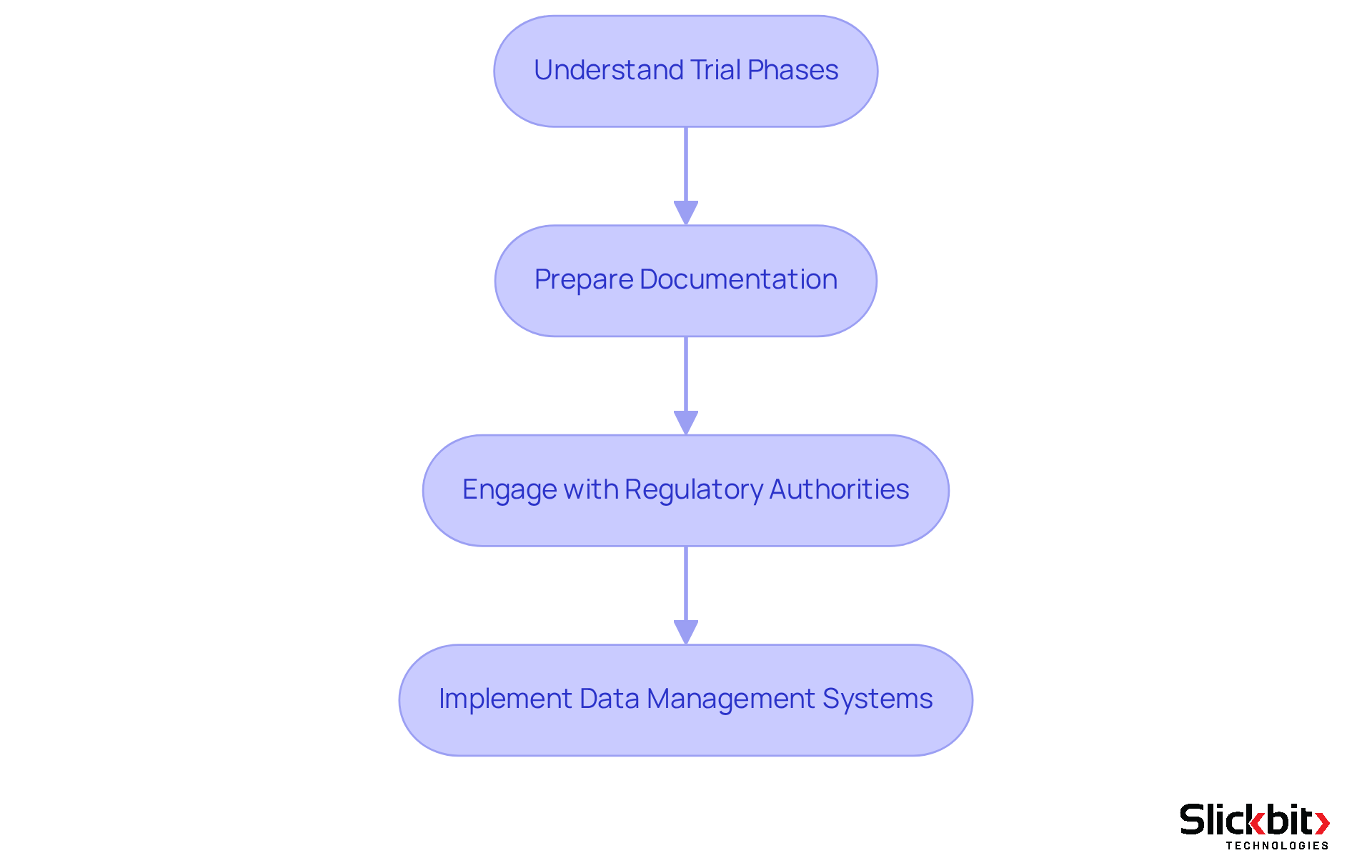
Manage Clinical Trials Within Regulatory Frameworks
To effectively manage clinical trials within regulatory frameworks, it is essential to consider the following steps:
-
Understand Trial Phases: Familiarize yourself with the distinct phases of clinical trials—Phase I, II, III, and IV—each with specific compliance requirements. Understanding these phases is crucial for adherence to drug regulatory affairs and for the successful execution of trials.
-
Prepare Documentation: Ensure that all necessary documentation, including the Clinical Trial Protocol and Informed Consent Forms, complies with legal standards. Compliance rates underscore the importance of meticulous documentation in drug regulatory affairs for securing official approval. Utilizing Slickbit's AI-driven Regulatory Intelligence can streamline this process by providing precise, traceable responses from FDA and international guidance documents, ensuring your documentation meets all required standards.
-
Engage with Regulatory Authorities: Maintain proactive communication with regulatory bodies throughout the trial duration. This engagement addresses concerns and clarifies expectations in drug regulatory affairs, fostering a collaborative relationship that can facilitate smoother approvals.
-
Implement Data Management Systems: Leverage AI-driven data management systems from Slickbit to monitor trial progress and uphold data integrity standards. These systems enhance efficiency by automating data gathering and verification processes, significantly reducing manual effort and improving adherence. Additionally, Vault Redact automates the identification and removal of PII and PHI from documents, ensuring compliance with regulatory requirements.
By efficiently overseeing clinical trials, you can enhance data quality and increase the likelihood of compliance, ultimately supporting the successful development of new therapies.
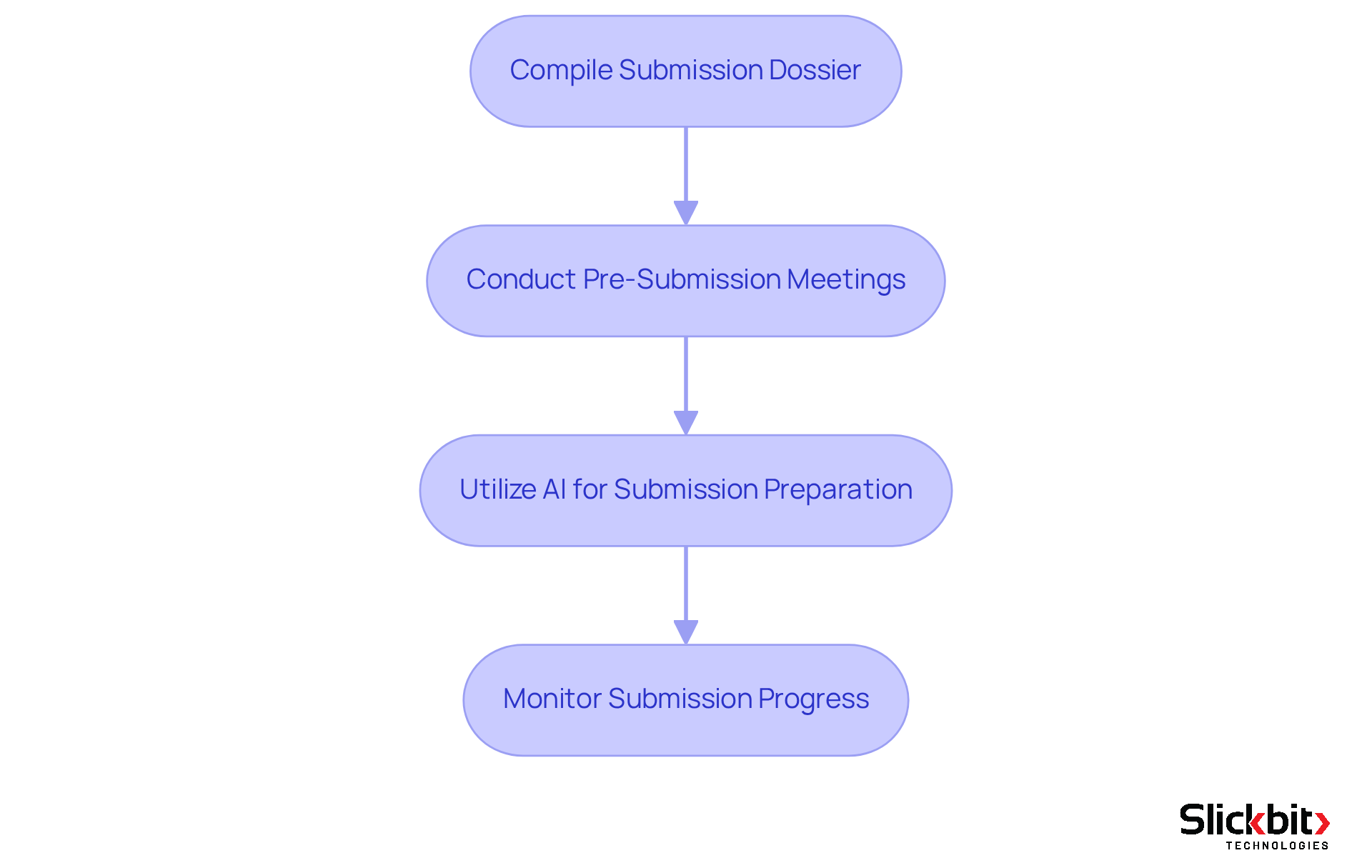
Facilitate Market Approval for New Drugs
To facilitate market approval for new drugs, it is imperative to follow these essential steps:
-
Compile Submission Dossier: Begin by gathering all necessary documents, including clinical trial data, manufacturing information, and labeling details, into a comprehensive submission dossier. This foundational step ensures that all relevant information is readily available for review.
-
Conduct Pre-Submission Meetings: Arrange meetings with oversight bodies to discuss your submission and address any potential issues. Engaging with regulatory agencies early on can clarify expectations and streamline the drug regulatory affairs approval process.
-
Utilize AI for Submission Preparation: Leverage AI tools, such as Trend 483, to enhance the preparation of your submission. These tools can identify trends in systemic risks and ensure adherence to compliance requirements. By improving the precision of your submission and offering deeper insights into FDA inspections, AI showcases its potential to streamline procedures in compliance matters and hospitality management.
-
Monitor Submission Progress: After submission, it is crucial to actively monitor the review process. Be prepared to respond promptly to any queries from regulatory agencies about drug regulatory affairs, demonstrating your commitment to compliance and transparency.
By diligently following these steps, you significantly enhance your chances of obtaining timely market approval for your new drug.
Conclusion
Mastering drug regulatory affairs is essential for R&D managers navigating the intricate landscape of pharmaceutical development. Understanding foundational concepts, ensuring compliance at every stage, managing clinical trials effectively, and facilitating market approval enables professionals to significantly enhance operational efficiency and contribute to the successful introduction of new therapies.
Key insights discussed in this guide emphasize:
- The importance of familiarity with regulatory agencies and terminology
- The necessity of a robust compliance strategy
- The role of AI tools in monitoring and managing compliance
- The value of proactive engagement with regulatory authorities
Each of these elements is vital in streamlining the drug development process and ensuring adherence to legal standards.
Ultimately, the significance of drug regulatory affairs extends beyond mere compliance; it fosters innovation while prioritizing public safety. R&D managers are encouraged to embrace these best practices and leverage modern tools to enhance their regulatory processes. By doing so, they position their organizations for success and contribute to the broader goal of delivering safe and effective medications to those in need.
Frequently Asked Questions
What is the importance of understanding drug regulatory affairs?
Understanding drug regulatory affairs is essential for mastering the field, as it involves knowledge of key concepts, regulatory agencies, and compliance requirements related to drug approval processes.
Which regulatory agencies oversee drug approval processes?
The main regulatory agencies are the FDA (Food and Drug Administration) and the EMA (European Medicines Agency), both of which ensure public safety through their oversight.
What are some key regulations in drug regulatory affairs?
Important regulations include the Food, Drug, and Cosmetic Act (FDCA) and the guidelines established by the European Medicines Agency (EMA).
What are some important terms to know in drug regulatory affairs?
Key terminology includes IND (Investigational New Drug), NDA (New Drug Application), and BLA (Biologics License Application).
How can understanding the basics of drug regulatory affairs improve expertise in the field?
By grasping the fundamentals, individuals can navigate the compliance environment more effectively, enhancing their knowledge and skills in drug regulatory affairs.




