Overview
This article addresses the critical strategies R&D managers must master concerning Corrective and Preventive Action (CAPA) within quality management in the pharmaceutical sector. The structured CAPA process is essential, particularly when enhanced by AI technologies, as it systematically tackles nonconformities. This approach not only improves product quality but also ensures regulatory compliance. Ultimately, it fosters a culture of continuous improvement that is vital for success in this highly regulated industry.
Introduction
Corrective and Preventive Action (CAPA) is essential in the pharmaceutical industry, especially within research and development, where high stakes and stringent regulatory scrutiny prevail. By accurately identifying and addressing deviations, CAPA not only rectifies existing issues but also strengthens processes against future risks, thereby safeguarding product quality and patient safety.
Nevertheless, mastering CAPA presents significant challenges, ranging from insufficient root cause analysis to the integration of advanced technologies. R&D managers must navigate these complexities to establish a robust CAPA framework that fosters continuous improvement and ensures regulatory compliance.
Define CAPA: Importance in Pharmaceutical R&D
Corrective and Preventive Action (CAPA) serves as a crucial framework within the pharmaceutical sector, systematically identifying, investigating, and addressing deviations or nonconformities in procedures, products, or systems. Its significance in research and development (R&D) is profound; CAPA not only rectifies existing issues but also implements measures to prevent their recurrence.
Efficient corrective and preventive actions enhance capa in quality, improve product quality, and guarantee adherence to regulations, ultimately protecting patient safety. Notably, shortcomings in CAPA have been associated with approximately 40% of Form 483 observations issued to pharmaceutical manufacturers, underscoring the urgent need for strong corrective and preventive action systems.
Furthermore, recent advancements in AI technology, such as Trend 483, enable organizations to leverage artificial intelligence to identify trends in systemic risks and repeat violations from FDA 483s. Trend 483 allows users to search, filter, and view full 483s directly for deeper insights, aiding in compliance and enhancing operational efficiency.
A well-organized corrective and preventive action strategy, supported by AI insights, enables companies to swiftly address manufacturing defects, significantly reducing the likelihood of product recalls and ensuring capa in quality with regulatory authorities and consumers. By fostering a culture of ongoing enhancement through corrective and preventive actions, supported by AI-driven insights, pharmaceutical companies can elevate their operational efficiency and ensure the integrity of their products in a highly regulated environment.
Outline the CAPA Process: Step-by-Step Implementation
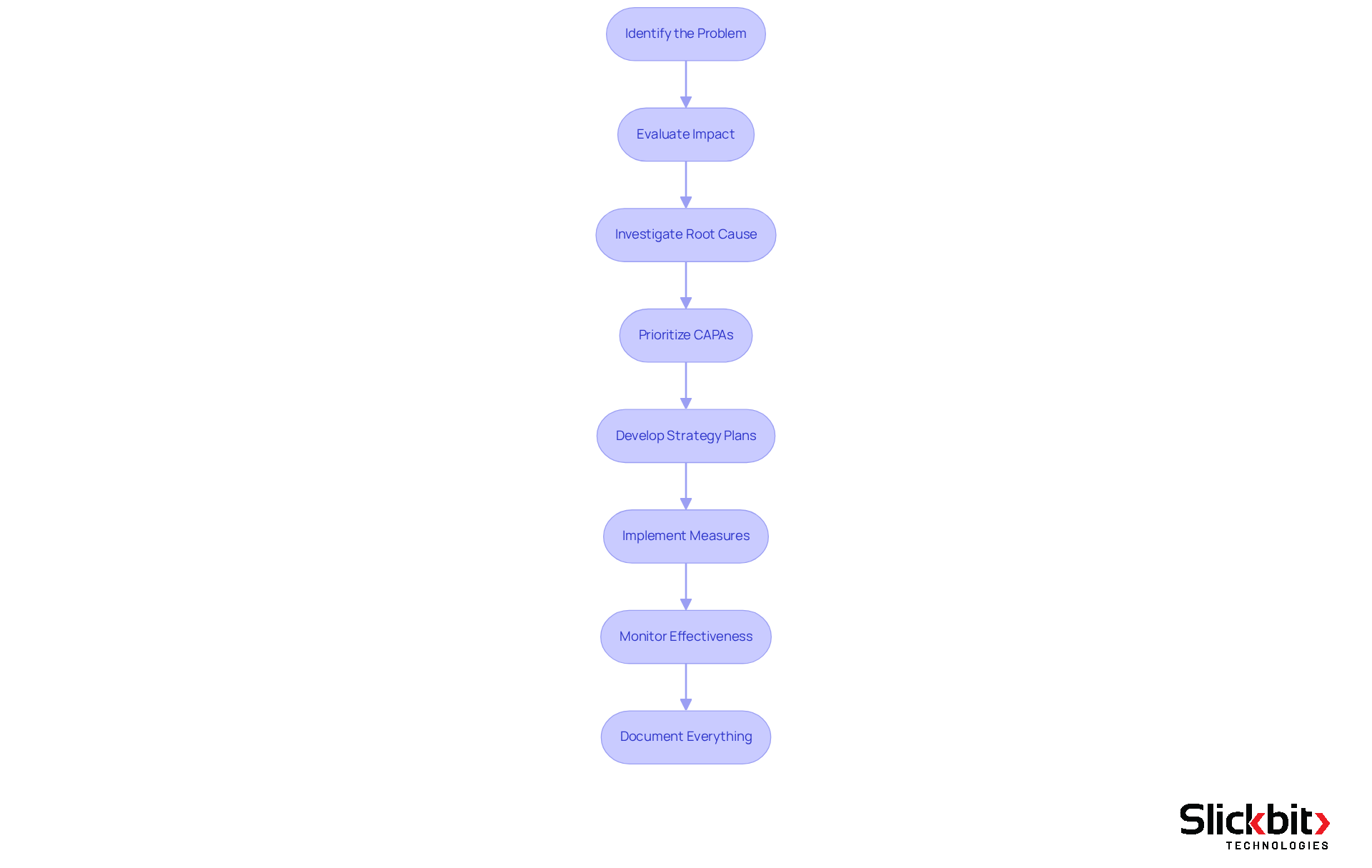
The CAPA process encompasses several essential steps that R&D managers must follow to ensure effective quality management:
- Identify the Problem: Recognize and document the issue needing attention, ensuring that all relevant details are captured.
- Evaluate the impact by assessing the severity and potential consequences of the problem on capa in quality, patient safety, and regulatory compliance.
- Investigate the Root Cause: Conduct a thorough investigation using tools like fishbone diagrams or the 5 Whys method to uncover the underlying causes of the issue.
- Prioritize CAPAs: Prioritize corrective and preventive measures based on risk severity, occurrence likelihood, and detection probability to ensure a strategic approach to CAPA management.
- Develop Strategy Plans: Formulate corrective and preventive strategy plans that directly address the identified root causes and outline steps to prevent recurrence.
- Implement Measures: Execute the plans while ensuring that all stakeholders are informed and engaged in the process.
- Monitor Effectiveness: Track the effectiveness of the implemented measures through Key Performance Indicators (KPIs) to confirm that the problem does not recur. This encompasses assessing the average duration required to resolve CAPAs, which is essential for ensuring adherence and operational efficiency.
- Document Everything: Keep thorough records of the corrective and preventive actions, including documentation of the issue, measures implemented, and verification outcomes, to ensure adherence and assist future audits.
By following these steps, R&D leaders can create a robust corrective and preventive action framework that not only enhances adherence to standards and capa in quality but also fosters a culture of continuous improvement within their organizations.
Integrate CAPA with Quality Management Systems for Enhanced Compliance
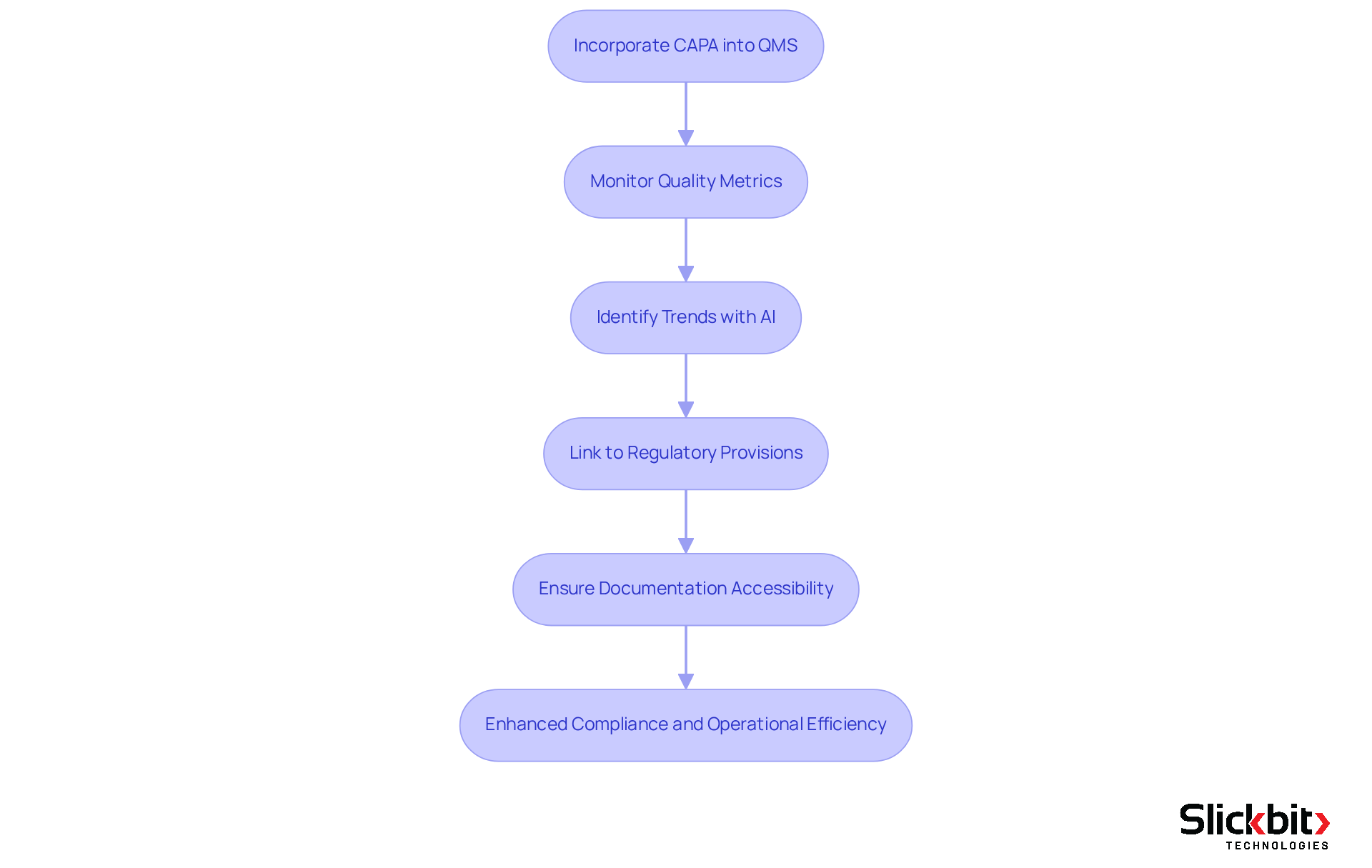
Incorporating Corrective and Preventive Measures within Quality Management Systems represents a strategic enhancement in adherence and operational effectiveness for pharmaceutical companies. By embedding corrective and preventive processes into a quality management system, organizations can systematically monitor and address remedial measures, ensuring swift and efficient execution. This integration facilitates real-time monitoring of quality metrics, enabling prompt responses to deviations and minimizing potential risks.
For example, AI-driven analytics from Slickbit's Trend 483 tool can identify trends in systemic risks and repeated violations from FDA 483s, while also allowing users to search, filter, and view complete 483s for deeper insights. Moreover, linking corrective and preventive actions to specific regulatory provisions, such as FDA 21 CFR 211.192, is essential for ensuring compliance during inspections.
A cohesive system guarantees that all documentation related to corrective and preventive actions is readily accessible, which is critical during audits and inspections. This holistic approach not only streamlines workflows but also fosters a culture of continuous improvement, empowering organizations to enhance their capa in quality management practices while maintaining regulatory compliance.
Furthermore, the integration of AI in hospitality solutions can further optimize operational efficiency, demonstrating the versatility of these technologies across various sectors.
Address Challenges in CAPA Implementation: Strategies for Success
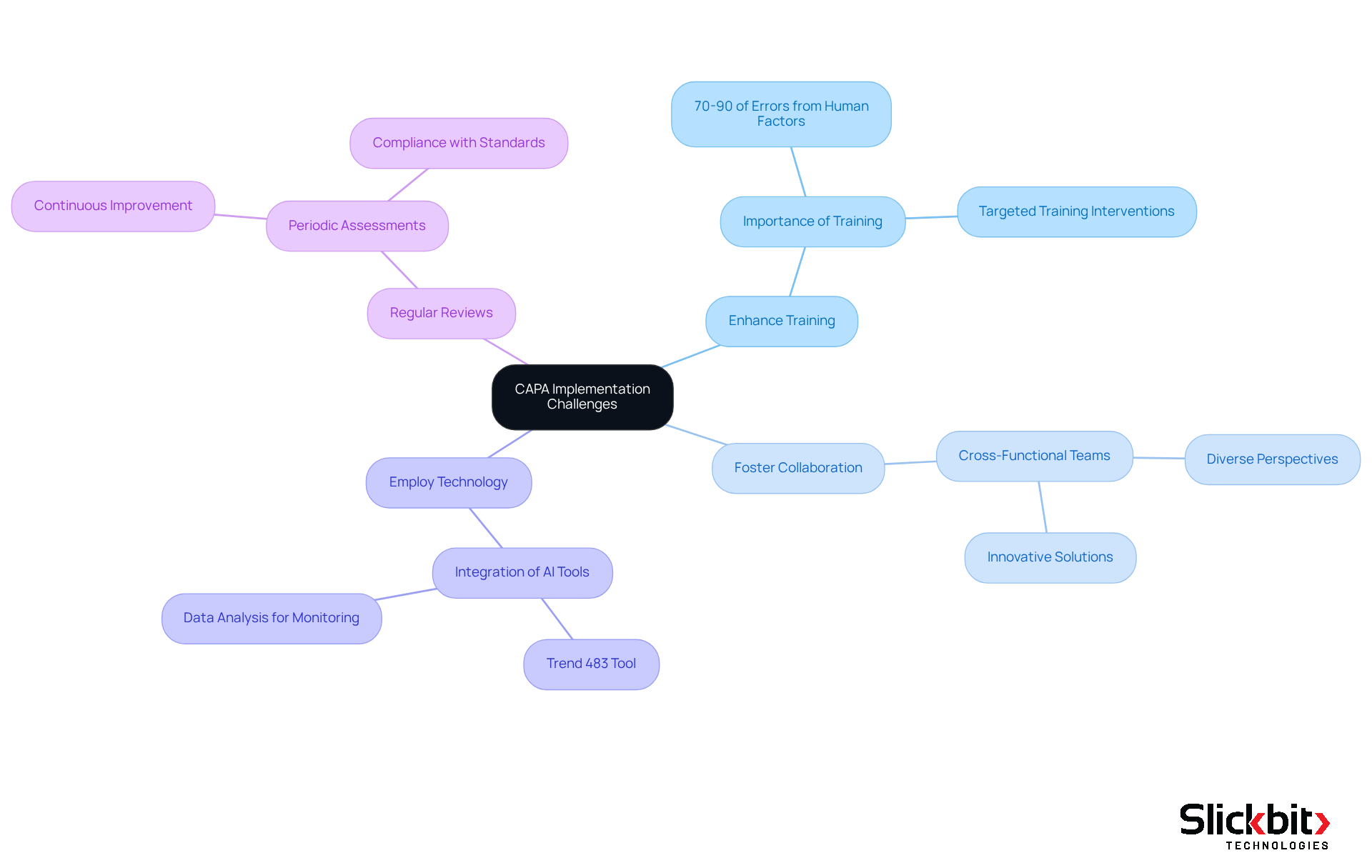
Establishing an efficient corrective and preventive action (CAPA) system, known as capa in quality, presents significant challenges, including inadequate root cause analysis, poor documentation practices, and insufficient cross-functional collaboration. To effectively address these obstacles, R&D managers can implement several strategic approaches.
-
Enhance Training: Comprehensive training for all team members involved in the CAPA process is vital. This training ensures that everyone understands their roles and responsibilities, which is essential for effective execution. Statistics indicate that 70-90% of workplace errors stem from human factors, underscoring the necessity for targeted training interventions.
-
Foster Collaboration: Encouraging cross-functional teams to engage in CAPA discussions can substantially enhance problem-solving capabilities. Diverse perspectives lead to more innovative solutions and a deeper understanding of issues, which are crucial for the effective management of corrective and preventive actions.
-
Employ Technology: The integration of AI and data analysis tools can streamline the CAPA process. These technologies facilitate the monitoring of issues and the analysis of trends, empowering organizations to proactively identify and address potential regulatory risks. For instance, Slickbit.ai's Trend 483 tool detects trends in systemic risks and regulatory challenges, enabling users to search, filter, and view comprehensive 483s for enhanced insights, thereby improving overall CAPA management.
-
Regular Reviews: Conducting periodic assessments of the CAPA process is essential for continuous improvement. These reviews help identify areas for enhancement and ensure that the system remains effective and compliant with evolving regulatory standards. A well-implemented Quality Management System (QMS) supports this by documenting preventive and corrective measures for audit purposes.
By proactively tackling these challenges, organizations can significantly enhance their CAPA in quality implementation, which ultimately drives continuous quality improvement and ensures compliance with regulatory requirements.
Conclusion
Mastering the Corrective and Preventive Action (CAPA) framework is essential for R&D managers in the pharmaceutical industry. It not only addresses existing issues but also lays the groundwork for preventing future occurrences. By embracing CAPA, organizations can significantly enhance product quality, ensure regulatory compliance, and ultimately safeguard patient safety.
This article outlines a comprehensive approach to CAPA, detailing its importance in identifying and rectifying deviations while fostering a culture of continuous improvement. Key strategies include:
- Implementing a structured CAPA process
- Integrating AI technologies for real-time insights
- Enhancing cross-functional collaboration
Each of these elements plays a pivotal role in creating a robust quality management system that meets industry standards and expectations.
To truly capitalize on the benefits of CAPA, organizations must remain proactive in addressing challenges such as inadequate root cause analysis and poor documentation practices. By investing in training, fostering collaboration, and leveraging technology, R&D managers can overcome these obstacles and drive significant improvements in quality management. Ultimately, the successful implementation of CAPA enhances operational efficiency and reinforces the commitment to excellence in the pharmaceutical sector, paving the way for safer and more effective products.
Frequently Asked Questions
What is CAPA in the pharmaceutical sector?
CAPA stands for Corrective and Preventive Action, a framework that systematically identifies, investigates, and addresses deviations or nonconformities in procedures, products, or systems within the pharmaceutical sector.
Why is CAPA important in pharmaceutical R&D?
CAPA is important in pharmaceutical R&D because it not only rectifies existing issues but also implements measures to prevent their recurrence, enhancing product quality and ensuring adherence to regulations, which ultimately protects patient safety.
What are the consequences of shortcomings in CAPA?
Shortcomings in CAPA have been linked to approximately 40% of Form 483 observations issued to pharmaceutical manufacturers, highlighting the need for robust corrective and preventive action systems.
How does AI technology enhance CAPA processes?
Recent advancements in AI technology, such as Trend 483, allow organizations to identify trends in systemic risks and repeat violations from FDA 483s, aiding in compliance and enhancing operational efficiency.
What benefits does a well-organized CAPA strategy provide?
A well-organized CAPA strategy, supported by AI insights, enables companies to quickly address manufacturing defects, reducing the likelihood of product recalls and ensuring quality compliance with regulatory authorities and consumers.
How can CAPA foster a culture of ongoing improvement in pharmaceutical companies?
By promoting ongoing enhancement through corrective and preventive actions and leveraging AI-driven insights, pharmaceutical companies can improve operational efficiency and maintain the integrity of their products in a highly regulated environment.




