Overview
This article delves into the effective integration of PTRS Pharma Solutions within research and development (R&D) processes, aiming to significantly enhance success rates in drug development. It presents a comprehensive step-by-step guide that underscores the critical importance of understanding key metrics such as:
- Probability of Technical Success (PTS)
- Regulatory Success (PRS)
Furthermore, it highlights the necessity of:
- Thorough assessments
- Proper training
- Continuous evaluation
to optimize R&D efficiency and ensure compliance. By following these guidelines, organizations can navigate the complexities of drug development with greater assurance and success.
Introduction
Navigating the intricacies of drug development can often resemble traversing a labyrinth, particularly given the dramatic variations in success rates across different stages. The Probability of Technical and Regulatory Success (PTS) and its counterpart, the Probability of Regulatory Success (PRS), serve as beacons of clarity within this complex landscape, guiding research and development teams toward more informed decision-making.
Consequently, how can organizations effectively integrate these critical metrics into their existing processes to enhance outcomes? This article presents a step-by-step guide for leveraging PTRS Pharma Solutions, uncovering strategies to optimize R&D efforts and ultimately elevate the chances of successfully bringing therapies to market.
Understand PTRS Pharma Solutions and Their Importance in R&D
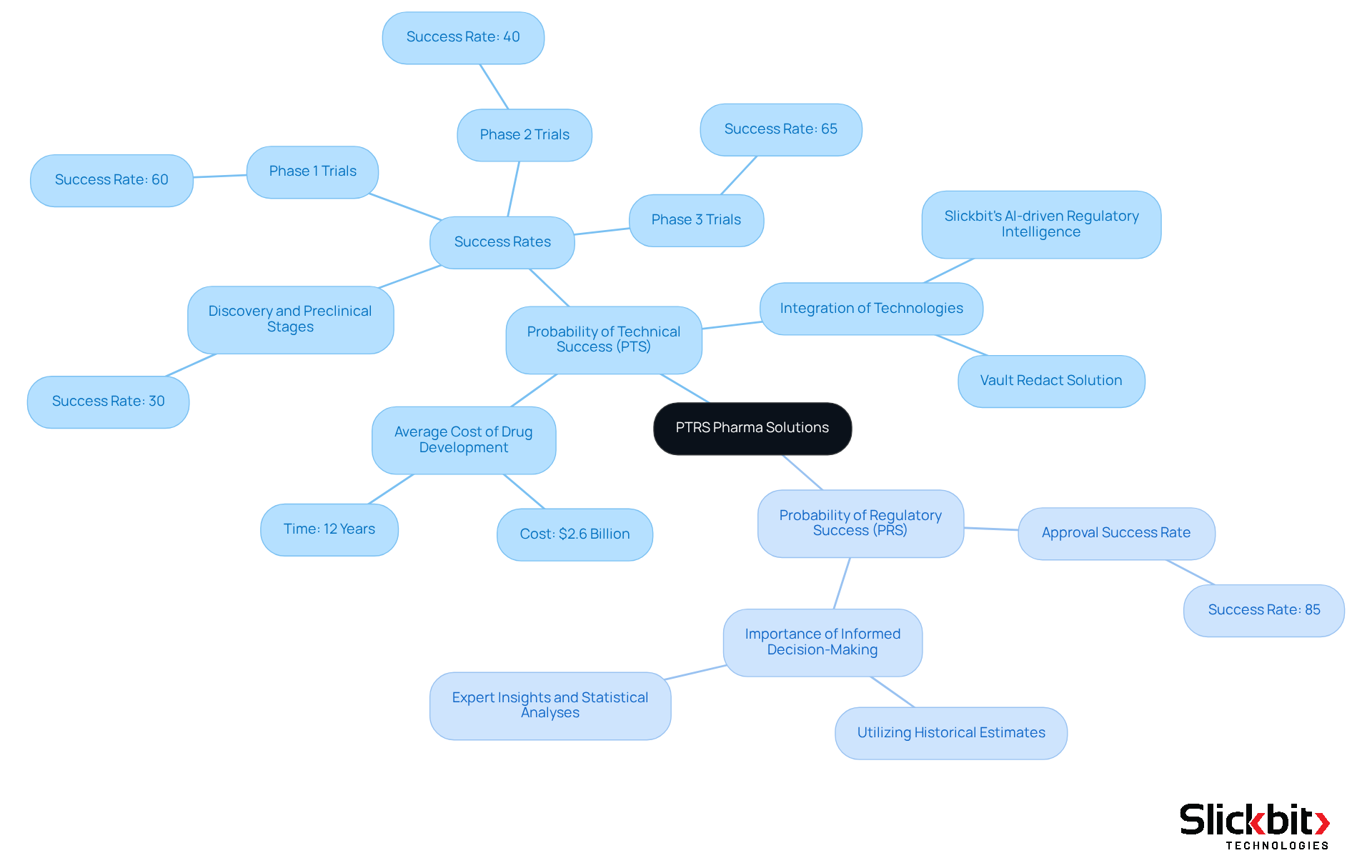
The Probability of Technical and Regulatory Success (PTS) is a vital metric that evaluates the likelihood of a candidate successfully navigating the various phases of development and ultimately securing regulatory approval. Understanding this system is crucial for research and development teams, as it aids in prioritizing projects, optimizing resource allocation, and making informed investment decisions. By effectively leveraging this system, organizations can enhance their strategic planning and risk management, leading to improved success rates in drug development.
Recent studies reveal that the success rate in the discovery and preclinical stages hovers around 30%, while Phase 3 trials exhibit a success rate of approximately 65%. This disparity underscores the importance of accurately assessing therapies at each stage, as the probability of success increases as treatments advance through the pipeline. Additionally, the average cost of bringing a drug from discovery to market is about $2.6 billion and takes roughly 12 years, highlighting the necessity for efficient resource allocation.
Organizations that have successfully integrated this system into their R&D processes showcase its tangible benefits. For instance, companies employing advanced AI and data analytics, such as Slickbit's AI-driven Regulatory Intelligence, can anticipate failures early in the drug development process. This technology provides precise, traceable insights from FDA and international guidance documents, enabling teams to eliminate candidates with subpar performance metrics and conserve valuable resources. Moreover, Slickbit's Vault Redact solution automates the identification and removal of PII and PHI from documents, facilitating compliance and boosting productivity in pharmaceutical development.
The Probability of Regulatory Success (PRS) represents another critical component of this system, boasting a regulatory approval success rate of approximately 85%. Understanding both PTS and PRS empowers R&D teams to navigate the complexities of drug development more effectively. By incorporating historical estimates, expert insights, and statistical analyses, organizations can refine their evaluations, ultimately leading to better-informed decisions regarding which candidates to advance.
In conclusion, familiarizing oneself with the key elements of the system, including PTS and PRS, is essential for recognizing its impact on R&D processes. As the pharmaceutical industry continues to evolve, the strategic implementation of innovative technologies from Slickbit will be pivotal in enhancing the success of drug development.
Assess Your Current R&D Processes for Integration Opportunities
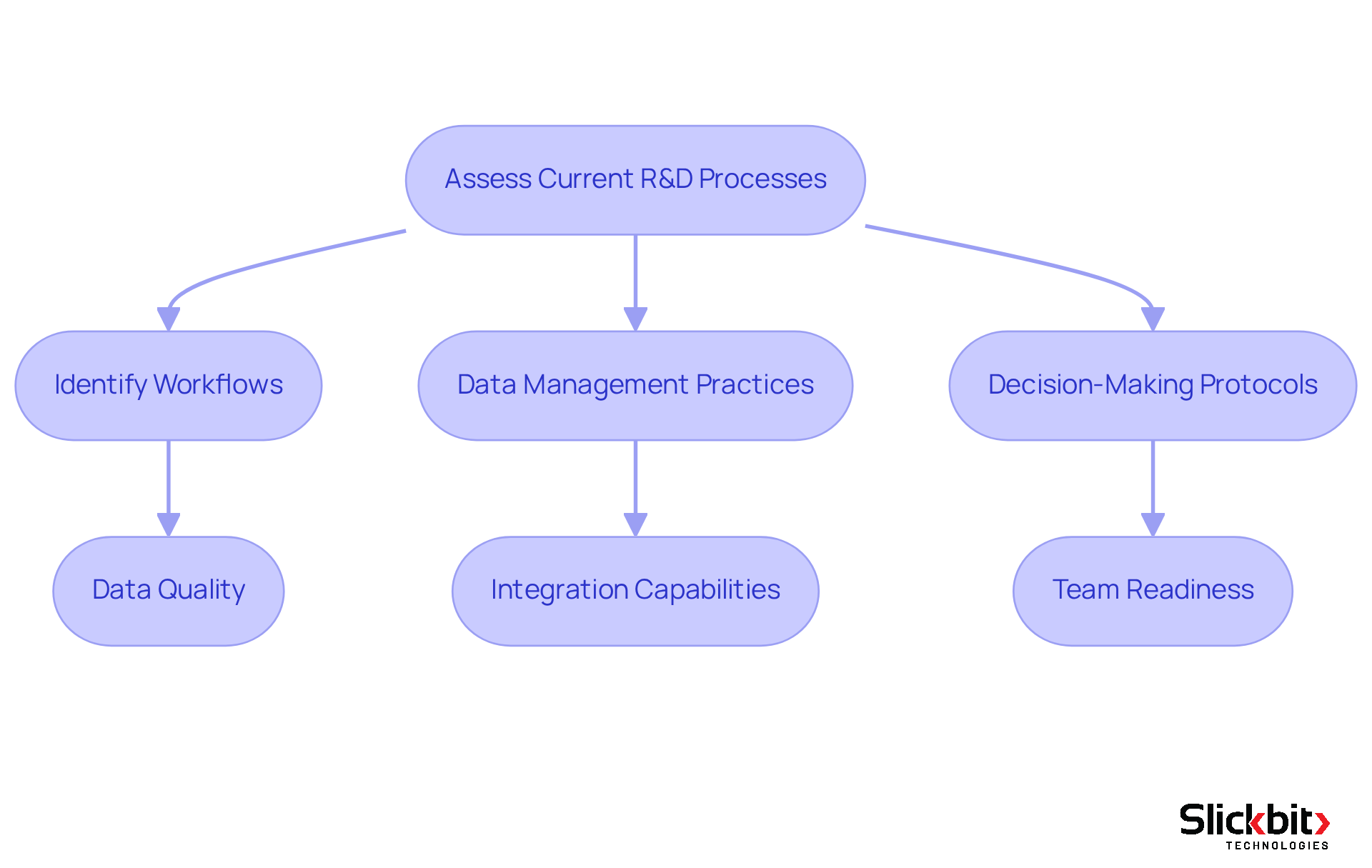
To successfully incorporate the proposed systems, begin with a thorough evaluation of your current research and development processes. Identify critical workflows, data management practices, and decision-making protocols. Engaging stakeholders from various departments is essential; their insights into pain points and inefficiencies are invaluable for understanding the operational landscape.
Furthermore, utilize process mapping tools to illustrate workflows, assisting in pinpointing specific areas where enhancements can significantly improve efficiency and ensure compliance. Key considerations during this assessment should encompass:
- Data quality
- Integration capabilities
- Your team's readiness to adopt new technologies
This comprehensive assessment will serve as a strategic guide for the effective execution of solutions, ultimately enhancing your R&D processes.
Implement PTRS Pharma Solutions: Step-by-Step Guide
-
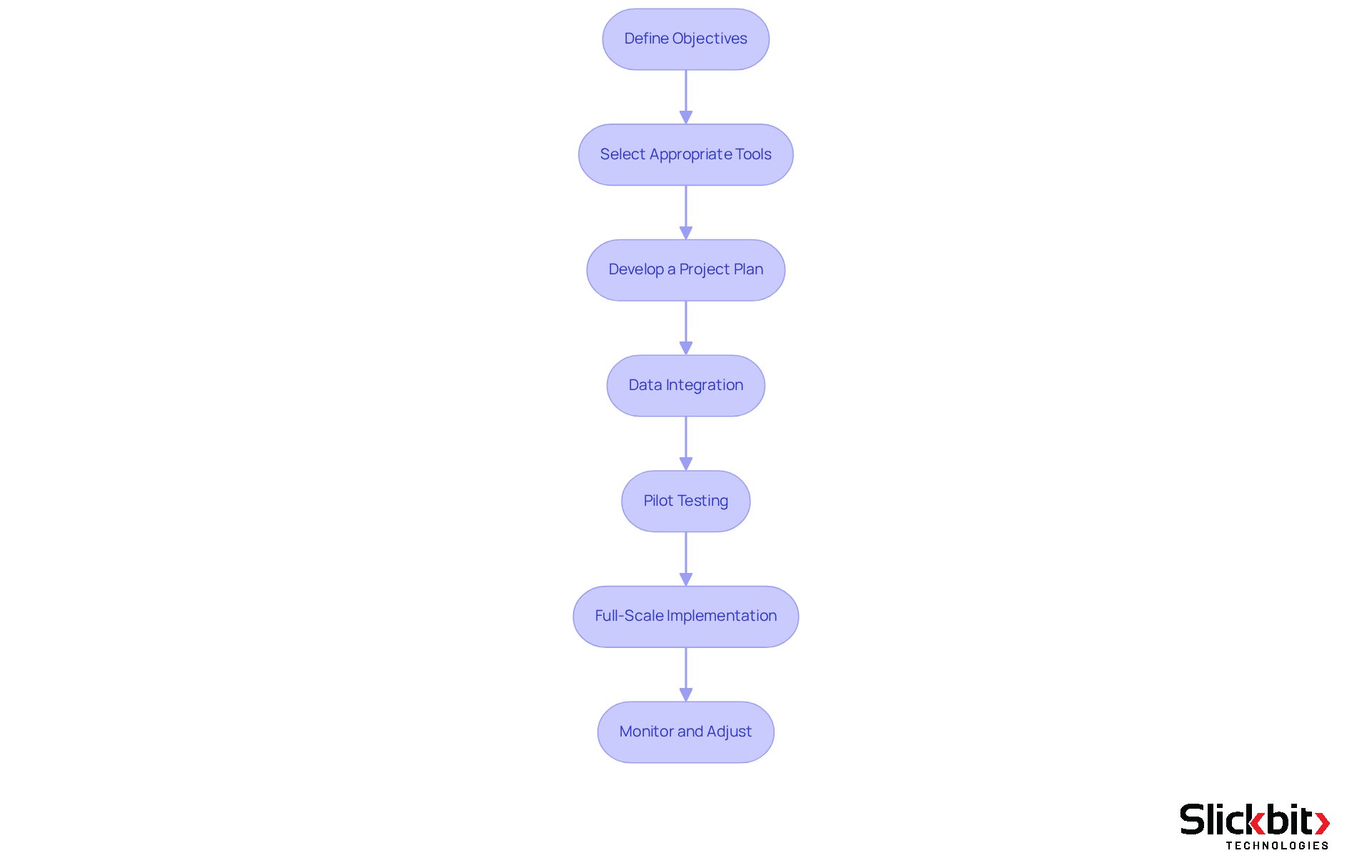
Define Objectives: Clearly outline the goals for the integration, such as enhancing decision-making processes and ensuring regulatory compliance. Establishing specific, measurable objectives is crucial for guiding the integration efforts. These objectives serve as a roadmap, ensuring that all stakeholders are aligned and focused on the desired outcomes.
-
Select Appropriate Tools: Choose software and tools that align with your R&D requirements and effectively support assessments related to ptrs pharma. Explore options such as Slickbit's Trend 483, which employs AI to detect patterns in systemic risks and compliance from FDA 483s. This choice guarantees that the tools can manage the intricacies of pharmaceutical development, ultimately enhancing operational efficiency.
-
Develop a Project Plan: Create a comprehensive project plan detailing timelines, responsibilities, and milestones for the integration process. Successful project plans often incorporate phased approaches that allow for iterative improvements and stakeholder engagement. Given that the average cost of developing a drug exceeds $2.6 billion, efficient project planning is essential to avoid costly delays and ensure timely delivery.
-
Data Integration: Combine current data sources into the system, concentrating on cleansing and normalizing data to guarantee precision. This step is vital, as the average time taken for full-scale implementation of new R&D tools can exceed 12 months. Therefore, data quality is paramount. Be aware of potential barriers to digital transformation, such as regulatory challenges and data integration issues, which can complicate this process and hinder progress.
-
Pilot Testing: Carry out a pilot evaluation of the system in a controlled setting to pinpoint possible problems and collect user input. This phase allows for adjustments before full-scale deployment, thereby minimizing disruptions and ensuring a smoother transition.
-
Full-Scale Implementation: Deploy the system across the organization, ensuring all groups receive adequate training and resources to utilize the new framework effectively. A well-executed implementation can significantly enhance operational efficiency and compliance, particularly with tools like Slickbit's AI-powered Regulatory Intelligence assistant, Lumino, which aids ptrs pharma in accessing accurate guidance and insights.
-
Monitor and Adjust: Continuously observe the performance of the system, making necessary modifications to enhance its effectiveness. Incorporate continuous monitoring and advanced analytics to ensure the system evolves with changing needs and remains effective, thereby sustaining long-term success.
Train Your Team for Successful Adoption of PTRS Solutions
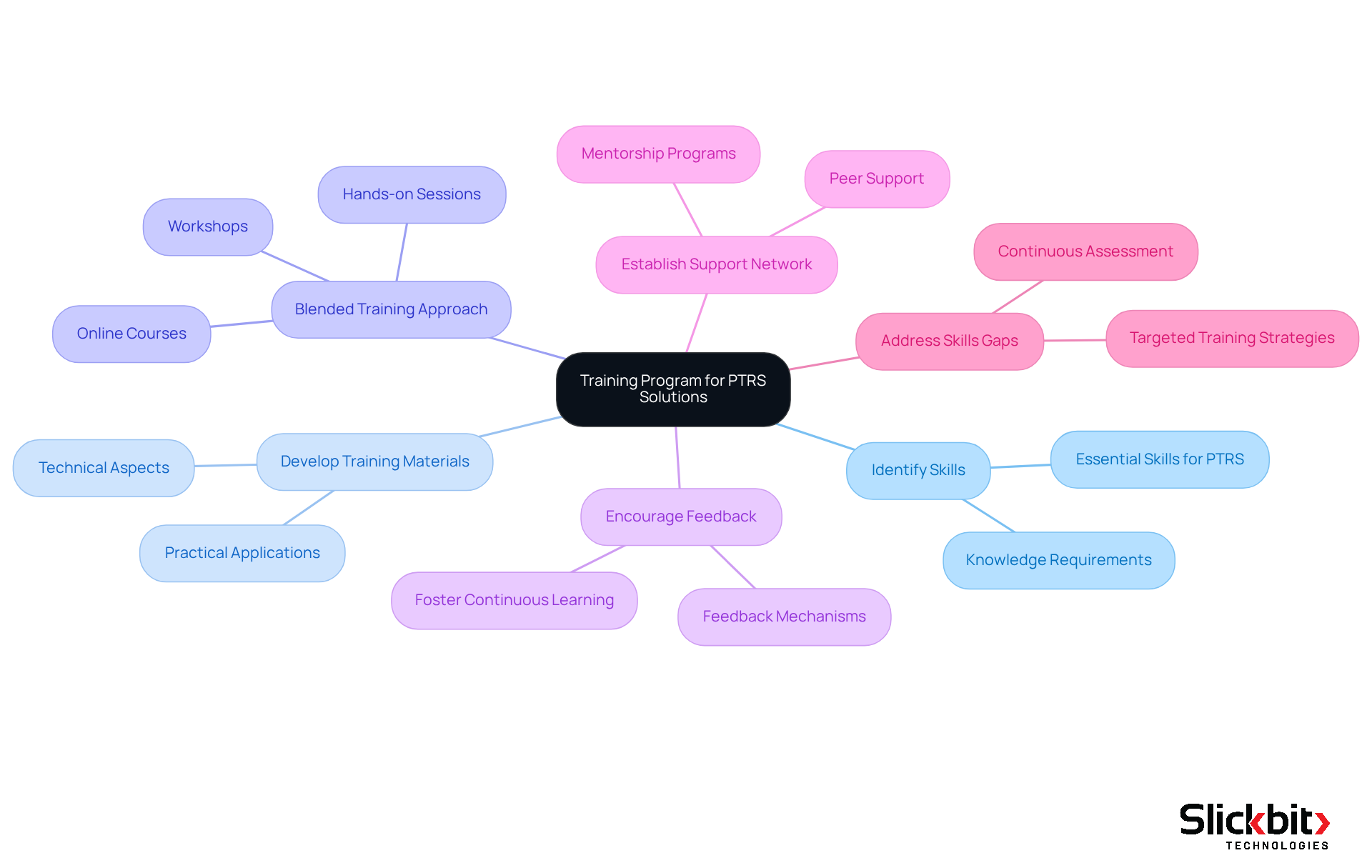
To ensure the effective integration of these systems, it is imperative to establish a comprehensive training program tailored to your group’s specific needs. Begin by identifying the essential skills and knowledge required for the proficient use of the new system. Develop training materials that encompass both the technical aspects of the ptrs pharma system and its practical applications within R&D workflows. A blended training approach is advisable, incorporating workshops, online courses, and hands-on sessions to accommodate diverse learning preferences.
Encouraging group members to ask questions and provide feedback during training fosters a culture of continuous learning, which is vital for successful technology adoption. Establishing a robust support network will also empower team members to seek assistance as they transition to utilizing the new tools. Notably, 80% of pharmaceutical manufacturers report a disconnect between current employee skills and evolving job requirements; thus, addressing these gaps through targeted training is essential. By investing in effective training strategies, organizations can enhance their workforce's capabilities, ultimately leading to improved outcomes in drug development and research.
Evaluate and Optimize Your R&D Process Post-Implementation
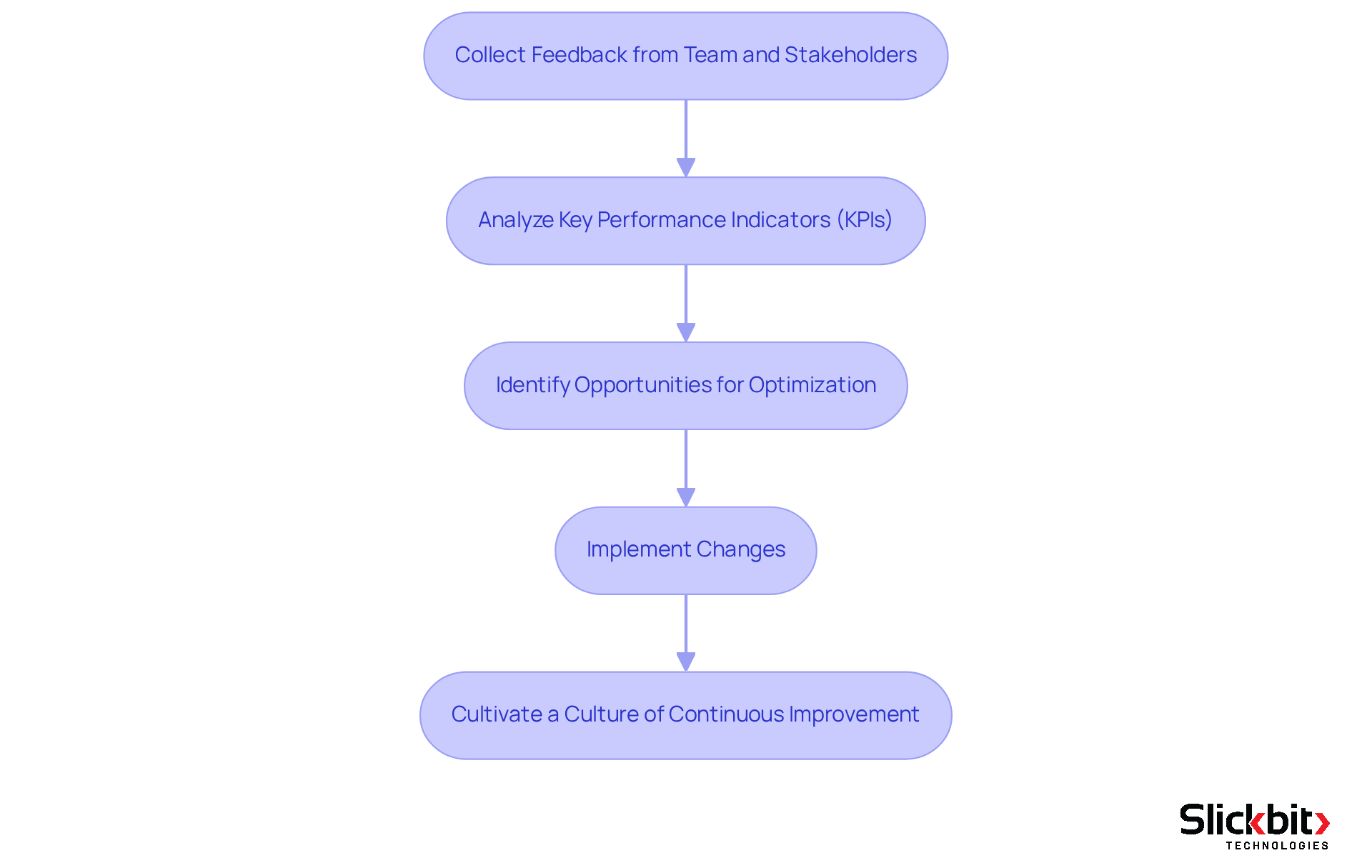
Following the implementation of the solutions, it is imperative to conduct a comprehensive evaluation of their impact on R&D processes. Begin by collecting feedback from team members and stakeholders to gauge the effectiveness of the integration. Analyze key performance indicators (KPIs) that reflect R&D efficiency, compliance, and success rates. Metrics such as the R&D Project On-Time Completion Rate and the Commercialization Success Rate offer critical insights into operational effectiveness.
Leverage this data to identify opportunities for optimization, such as refining workflows or enhancing data management practices. Regular reviews of your strategies are essential to ensure alignment with organizational objectives and industry standards. Furthermore, utilizing AI tools like Slickbit's Trend 483 can significantly enhance this evaluation by identifying trends in systemic risks and compliance issues, thereby facilitating more informed decision-making.
Cultivating a culture of continuous improvement is vital; by promoting this mindset, you can maximize the advantages of PTRS Pharma solutions and foster sustained success in your R&D initiatives. This proactive approach not only boosts efficiency but also positions your organization to adapt to shifting market demands and regulatory landscapes.
Conclusion
Integrating PTRS Pharma Solutions into research and development processes is not merely a strategic enhancement; it represents a transformative approach that significantly increases the likelihood of success in drug development. By grasping the Probability of Technical and Regulatory Success (PTS) and the Probability of Regulatory Success (PRS), organizations can make informed decisions that prioritize projects and optimize resource allocation effectively. This comprehensive understanding establishes a foundation for improved strategic planning and risk management within the pharmaceutical landscape.
Throughout the article, key insights have been illuminated, underscoring the critical need for thorough assessments of existing R&D processes, the step-by-step implementation of PTRS solutions, and the importance of training teams for successful adoption. Emphasizing the integration of advanced technologies, such as AI-driven tools, not only streamlines workflows but also enhances compliance and decision-making capabilities. The value of continuous evaluation and optimization post-implementation further ensures that organizations remain agile and responsive to evolving market demands and regulatory environments.
In conclusion, embracing PTRS Pharma Solutions is essential for organizations striving to thrive in the competitive pharmaceutical industry. By committing to a structured integration process and fostering a culture of continuous improvement, companies can significantly enhance their R&D outcomes. It is imperative for industry leaders to recognize the importance of these systems and invest in the necessary training and evaluation strategies to maximize their potential. Ultimately, this commitment will lead to more successful drug development initiatives and improved patient outcomes.
Frequently Asked Questions
What is the Probability of Technical and Regulatory Success (PTS) and why is it important?
The Probability of Technical and Regulatory Success (PTS) is a metric that evaluates the likelihood of a candidate successfully navigating development phases and securing regulatory approval. It is crucial for R&D teams as it helps prioritize projects, optimize resource allocation, and make informed investment decisions.
What are the success rates at different stages of drug development?
Recent studies indicate that the success rate in the discovery and preclinical stages is around 30%, while Phase 3 trials have a success rate of approximately 65%. This highlights the importance of accurately assessing therapies at each stage.
What is the average cost and time required to bring a drug to market?
The average cost of bringing a drug from discovery to market is about $2.6 billion, and it typically takes roughly 12 years.
How can organizations enhance their R&D processes using technology?
Organizations can enhance their R&D processes by integrating advanced technologies like AI and data analytics. For example, Slickbit's AI-driven Regulatory Intelligence can help anticipate failures early in the drug development process, allowing teams to eliminate underperforming candidates and conserve resources.
What is the Probability of Regulatory Success (PRS) and its significance?
The Probability of Regulatory Success (PRS) is another critical component of the system, with a regulatory approval success rate of approximately 85%. Understanding both PTS and PRS helps R&D teams navigate drug development complexities more effectively.
What steps should organizations take to integrate new systems into their R&D processes?
Organizations should begin with a thorough evaluation of their current R&D processes, identifying critical workflows and decision-making protocols. Engaging stakeholders for insights on pain points and utilizing process mapping tools can help pinpoint areas for improvement.
What key considerations should be included in the assessment of current R&D processes?
Key considerations should include data quality, integration capabilities, and the team's readiness to adopt new technologies. This assessment will guide the effective execution of solutions to enhance R&D processes.




