Overview
This article delves into the critical integration of current Good Manufacturing Practices (cGMP) into pharmaceutical research and development (R&D) workflows. The significance of cGMP cannot be overstated; it is essential for ensuring product safety and quality. To facilitate compliance, a step-by-step process is outlined, which includes:
- Assessing current practices
- Developing adherence plans
- Training personnel
- Leveraging technology to enhance regulatory compliance
By following these guidelines, organizations can effectively navigate the complexities of pharmaceutical manufacturing while upholding the highest standards of safety and quality. Ultimately, the adoption of cGMP not only protects consumers but also reinforces the credibility of the pharmaceutical industry as a whole.
Introduction
The pharmaceutical industry operates under rigorous standards to ensure the safety and efficacy of its products, with Current Good Manufacturing Practices (cGMP) serving as a cornerstone of research and development. By understanding and integrating cGMP, organizations can significantly enhance product quality and mitigate risks associated with drug development. However, as regulations evolve and compliance complexities increase, R&D teams face the challenge of navigating this landscape effectively.
How can they ensure adherence while fostering innovation? This guide presents a comprehensive, step-by-step approach to seamlessly incorporate cGMP into pharmaceutical workflows, paving the way for improved patient outcomes and regulatory success.
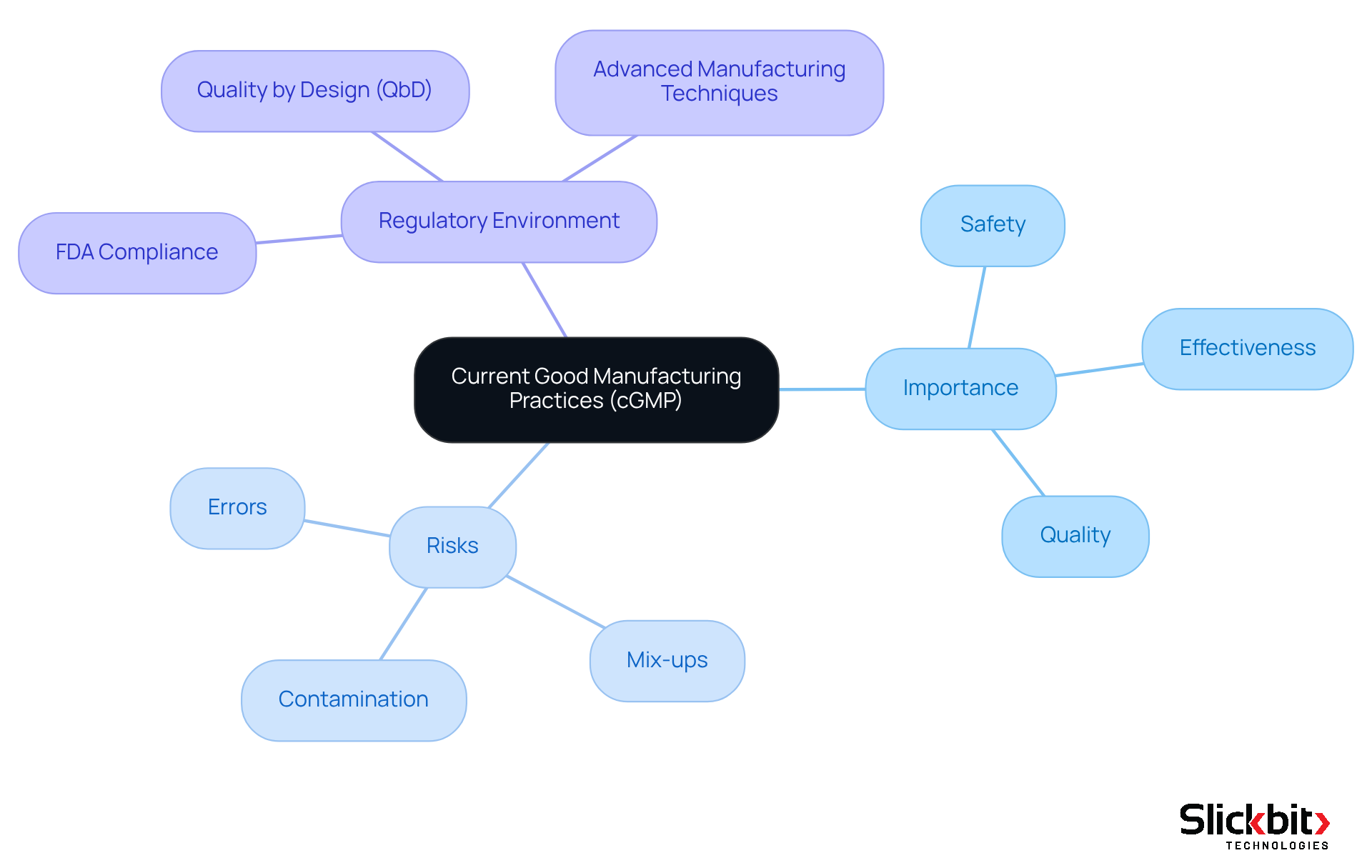
Define cGMP and Its Importance in Pharmaceutical R&D
Current Good Manufacturing Practices (c gmp) encompass the regulations established by the FDA and other regulatory agencies to ensure that pharmaceutical products are consistently manufactured and regulated according to stringent standards. The significance of c gmp in pharmaceutical research and development cannot be overstated; it is essential for ensuring that products are safe, effective, and of the highest quality.
Adhering to c gmp mitigates risks associated with drug development, such as:
- Contamination
- Mix-ups
- Errors
These risks can lead to serious patient safety issues and regulatory non-compliance. Notably, the majority of firms evaluated by the FDA are found to be fully compliant with good manufacturing practices, underscoring the effectiveness of these protocols in maintaining pharmaceutical standards.
Furthermore, the FDA's emphasis on advanced manufacturing techniques and Quality by Design (QbD) principles reflects a pivotal shift within the industry towards more robust quality management systems. As we approach 2025, the FDA continues to underscore the importance of c gmp in drug development, with regulatory measures aimed at preventing unsafe or ineffective medications.
Understanding good manufacturing practices is crucial for research and development teams to align their processes with industry standards and regulatory expectations, ultimately leading to improved patient outcomes and enhanced compliance.
Identify Key Regulatory Requirements for cGMP Compliance
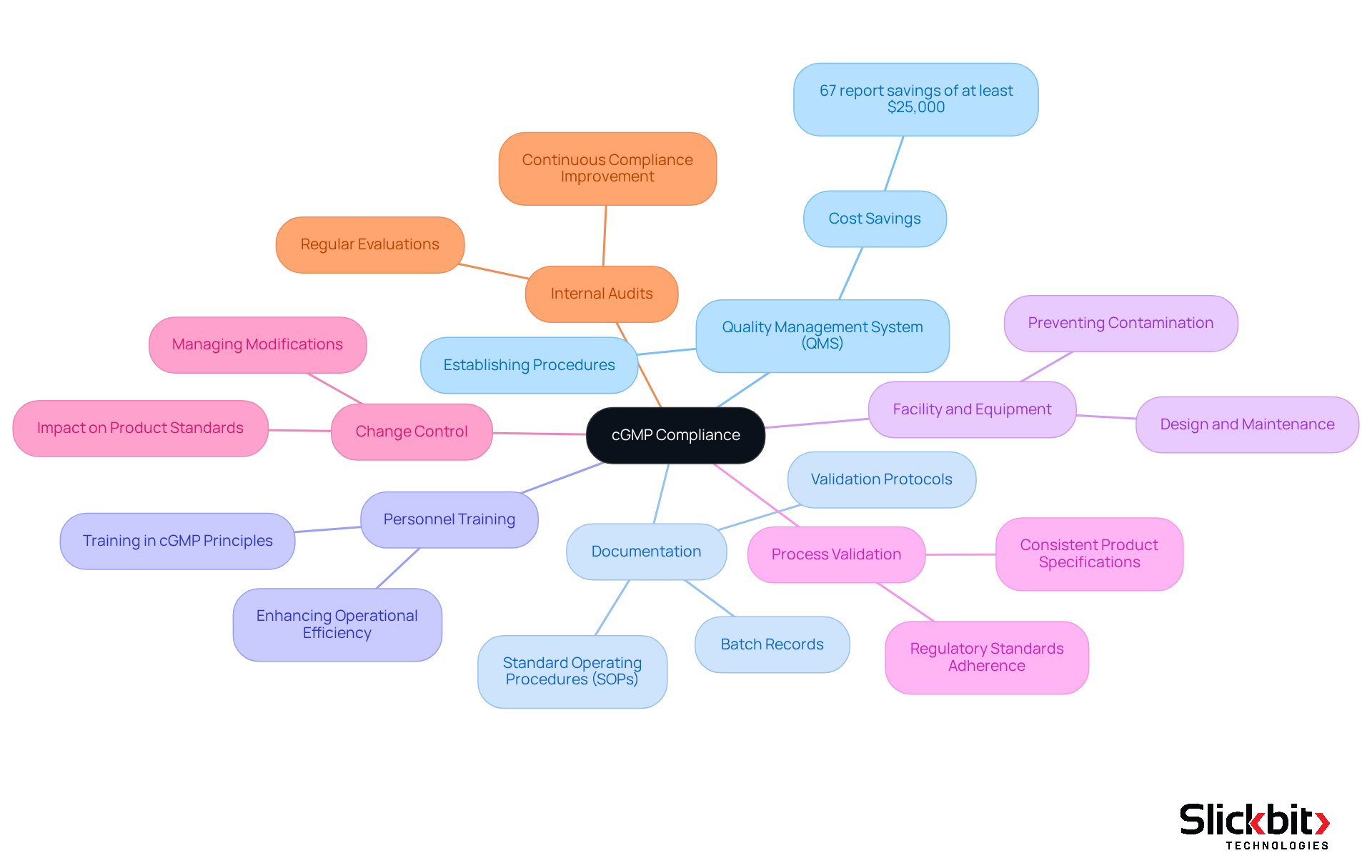
To achieve c gmp compliance, pharmaceutical R&D teams must follow several key regulatory requirements, which are essential for ensuring product quality and maintaining regulatory adherence.
-
Quality Management System (QMS): Establishing a robust QMS is essential, as it outlines procedures for assurance and control. Organizations with effective QMS can achieve significant savings; notably, 67% report at least $25,000 in savings within the first year of implementation.
-
Documentation: Maintaining accurate and thorough documentation of all processes is essential. This includes batch records, standard operating procedures (SOPs), and validation protocols, which are vital for demonstrating adherence to regulations and ensuring product quality.
-
Personnel Training: Ensuring that all staff are adequately trained in c gmp principles and practices is critical. A well-prepared workforce is fundamental for upholding regulations and enhancing operational efficiency.
-
Facility and Equipment: It is necessary to design and maintain facilities and equipment that meet c gmp standards. This involves ensuring cleanliness and proper maintenance to prevent contamination and safeguard product integrity.
-
Process Validation: Validating manufacturing processes is essential to ensure they consistently produce products that meet predetermined specifications. This step is vital for maintaining adherence to regulatory standards.
-
Change Control: Implementing a change control system is important for managing modifications to processes, equipment, or materials. This ensures that any changes do not adversely affect product standards or compliance with regulations.
-
Internal Audits: Performing regular internal evaluations is essential to assess adherence to c gmp regulations and identify areas for improvement. These audits serve as a proactive measure to ensure continuous compliance with standards and regulatory requirements.
By concentrating on these essential areas, pharmaceutical R&D teams can effectively integrate current Good Manufacturing Practices into their operations, ultimately enhancing product quality and ensuring regulatory compliance.
Integrate cGMP into Your R&D Workflows: Step-by-Step Process
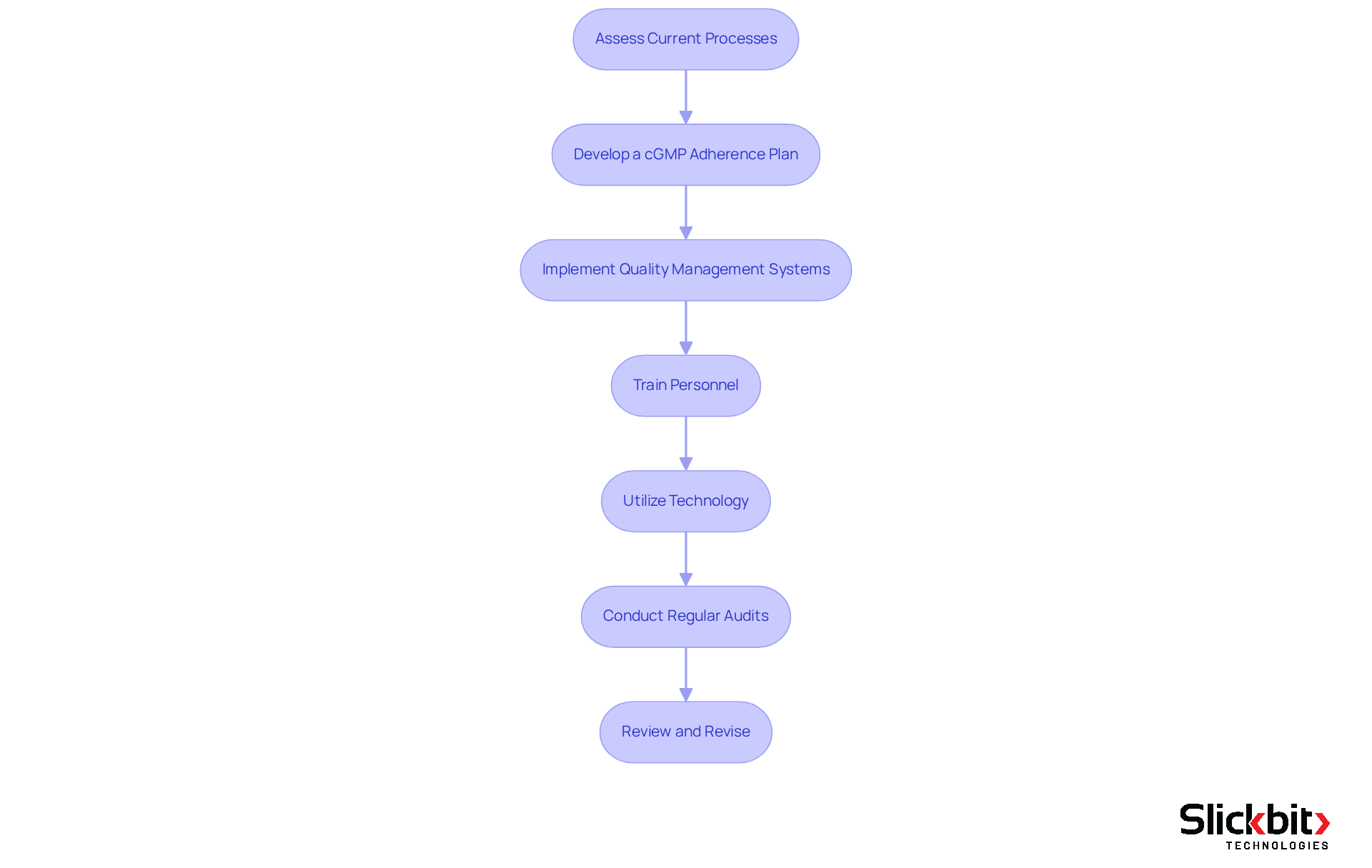
Integrating cGMP into R&D workflows requires a strategic approach that encompasses several key steps:
-
Assess Current Processes: Begin by conducting a thorough evaluation of existing R&D processes to identify gaps in adherence to cGMP standards. This evaluation must consider the most recent FDA guidelines and the historical development of cGMP, which emphasizes rigorous control standards.
-
Develop a cGMP Adherence Plan: Formulate a comprehensive adherence strategy that addresses identified gaps, detailing timelines and assigning responsibilities. This plan should reflect the flexibility permitted by the FDA in defining significant phases of the manufacturing process, ensuring scientific justification for each step.
-
Implement Quality Management Systems: Establish a robust Quality Management System (QMS) that encompasses Standard Operating Procedures (SOPs), documentation practices, and quality control measures. Effective QMS practices are essential for upholding regulations and minimizing risks in clinical trials.
-
Train Personnel: Arrange training sessions for all team members on current good manufacturing practices and their specific responsibilities in upholding standards. Ongoing education is crucial, as companies that emphasize cGMP are better equipped to handle regulatory challenges and achieve successful market entry.
-
Utilize Technology: Leverage advanced AI tools, such as Slickbit's Trend 483, to automate documentation, monitor adherence metrics, and streamline workflows. This tool can identify trends in systemic risks and repeat violations from FDA 483s, significantly enhancing the efficiency of regulatory processes and reducing the likelihood of errors and contamination. Furthermore, Trend 483 enables users to search, filter, and view complete 483s directly for deeper insights into regulatory processes.
-
Conduct Regular Audits: Schedule periodic audits to ensure ongoing compliance and identify areas for improvement. Regular audits are essential for maintaining high standards and adapting to evolving regulatory requirements.
-
Review and Revise: Continuously assess and update procedures and educational resources to reflect changes in regulations and best practices in cGMP compliance. This iterative method guarantees that your R&D processes remain in accordance with current good manufacturing practices, ultimately improving product quality and safety.
Provide Training and Resources for Effective cGMP Implementation
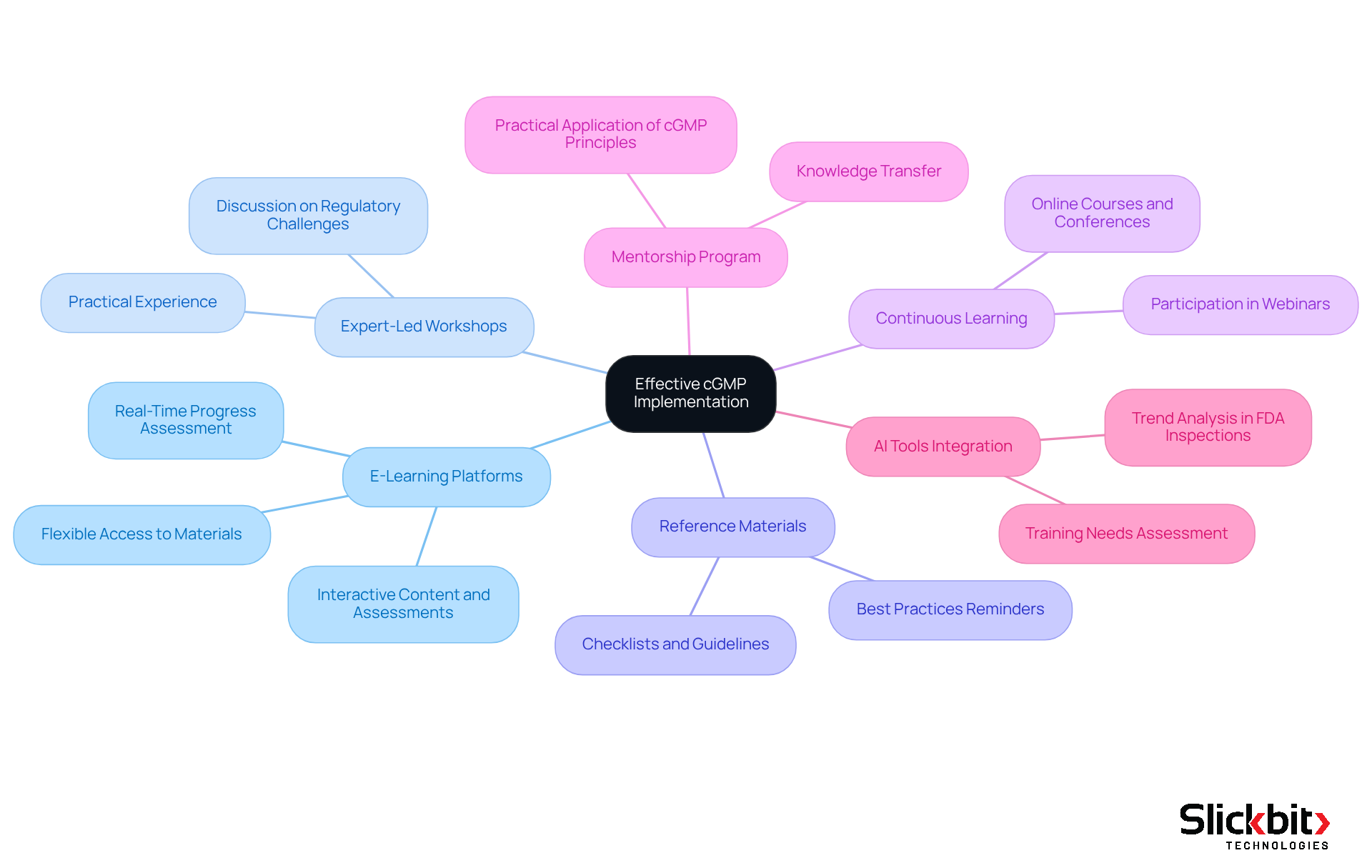
To ensure effective c gmp implementation, organizations must prioritize comprehensive training and resources.
Organizations should develop customized learning programs that encompass c gmp principles, regulatory requirements, and the specific responsibilities of various roles within the organization. This approach ensures that all employees understand their compliance obligations and are equipped to meet them effectively.
-
Utilize E-Learning Platforms: Implementing e-learning platforms provides employees with flexible access to educational materials, allowing them to learn at their own pace. Such platforms enhance engagement and retention through interactive content and assessments. Furthermore, organizations should assess employees' progress in real-time to evaluate the effectiveness of the instruction, ensuring that it is impactful and meets their needs.
-
Conducting expert-led workshops organized by c gmp specialists offers employees practical experience and discussions on real-world regulatory challenges. This hands-on approach enables employees to apply their theoretical knowledge to actual scenarios, solidifying their understanding of compliance.
-
Distribute Reference Materials: Providing accessible reference materials, such as checklists and guidelines, supports daily operations and reinforces training concepts. These resources serve as quick reminders of best practices and compliance standards, ensuring that employees can easily reference essential information.
-
Encourage Continuous Learning: Cultivating a culture of ongoing education motivates employees to stay informed about c gmp and industry best techniques. Encouraging participation in webinars, online courses, and industry conferences fosters a sense of engagement, making learning more enjoyable and effective.
-
Establish a Mentorship Program: Pairing seasoned employees with newer team members facilitates knowledge transfer and practical application of c gmp principles. This mentorship fosters a nurturing educational atmosphere and enhances team unity, aligning with the aim of continuous learning and efficient adherence to compliance standards.
-
Integrate AI Tools for Enhanced Adherence: Utilizing AI tools, such as Slickbit's Trend 483, helps organizations identify trends in FDA inspections and improve regulatory monitoring. These tools enable organizations to assess training needs based on inspection data, ensuring that training programs are aligned with current compliance challenges.
Conclusion
Integrating Current Good Manufacturing Practices (cGMP) into pharmaceutical research and development is essential for ensuring product quality, safety, and regulatory compliance. This integration not only protects patient health but also enhances operational efficiency and mitigates risks associated with drug development. By prioritizing cGMP, organizations can adeptly navigate the complexities of regulatory requirements while fostering a culture of quality throughout their R&D processes.
This article outlines the critical steps necessary for the successful integration of cGMP. It emphasizes the importance of:
- Assessing current workflows
- Developing adherence plans
- Implementing robust quality management systems
Training personnel, utilizing technology, and conducting regular audits are vital to ensuring ongoing compliance. Furthermore, the focus on continuous learning and resource availability guarantees that teams remain informed and equipped to effectively tackle compliance challenges.
Ultimately, adherence to cGMP transcends mere regulatory obligation; it represents a commitment to excellence in pharmaceutical development. Organizations are urged to proactively adopt these practices, as they not only safeguard public health but also enhance the credibility and success of pharmaceutical innovations. By embracing cGMP principles, the industry can continue to deliver safe and effective medications that meet the highest standards of quality.
Frequently Asked Questions
What does cGMP stand for?
cGMP stands for Current Good Manufacturing Practices, which are regulations established by the FDA and other regulatory agencies.
Why is cGMP important in pharmaceutical research and development?
cGMP is essential for ensuring that pharmaceutical products are consistently manufactured and regulated according to stringent standards, ensuring they are safe, effective, and of the highest quality.
What risks does adherence to cGMP help mitigate in drug development?
Adherence to cGMP helps mitigate risks such as contamination, mix-ups, and errors, which can lead to serious patient safety issues and regulatory non-compliance.
How compliant are pharmaceutical firms with cGMP regulations?
The majority of firms evaluated by the FDA are found to be fully compliant with good manufacturing practices, indicating the effectiveness of these protocols in maintaining pharmaceutical standards.
What recent shifts have occurred in the FDA's approach to manufacturing practices?
The FDA has emphasized advanced manufacturing techniques and Quality by Design (QbD) principles, reflecting a shift towards more robust quality management systems.
What is the FDA's focus regarding cGMP as we approach 2025?
The FDA continues to emphasize the importance of cGMP in drug development, with regulatory measures aimed at preventing unsafe or ineffective medications.
Why is understanding cGMP crucial for research and development teams?
Understanding cGMP is crucial for research and development teams to align their processes with industry standards and regulatory expectations, ultimately leading to improved patient outcomes and enhanced compliance.




