Overview
This article presents a comprehensive step-by-step guide for implementing business intelligence (BI) within pharmaceutical research and development (R&D), aimed at significantly enhancing efficiency and decision-making processes. By integrating BI tools such as Tableau and Power BI, organizations can streamline their operations and improve outcomes.
The article meticulously outlines structured implementation steps, underscoring the critical role of monitoring performance through key performance indicators (KPIs). This approach is essential to ensure successful results in drug development processes, ultimately driving innovation and effectiveness in the industry.
Introduction
In the dynamic realm of pharmaceutical research and development, the incorporation of business intelligence (BI) technologies is not merely a trend; it is essential for maintaining a competitive edge. By leveraging data-driven insights, organizations can optimize operations, improve compliance, and expedite drug development processes.
Nevertheless, the challenge resides in the effective integration of these advanced tools into existing workflows. How can pharmaceutical companies adeptly navigate this intricate landscape to utilize BI for enhanced outcomes and quicker patient access to groundbreaking treatments?
This guide explores the critical steps for successfully embedding business intelligence into pharmaceutical R&D, emphasizing the tools and strategies that have the potential to revolutionize the industry.
Understand Business Intelligence in Pharmaceuticals
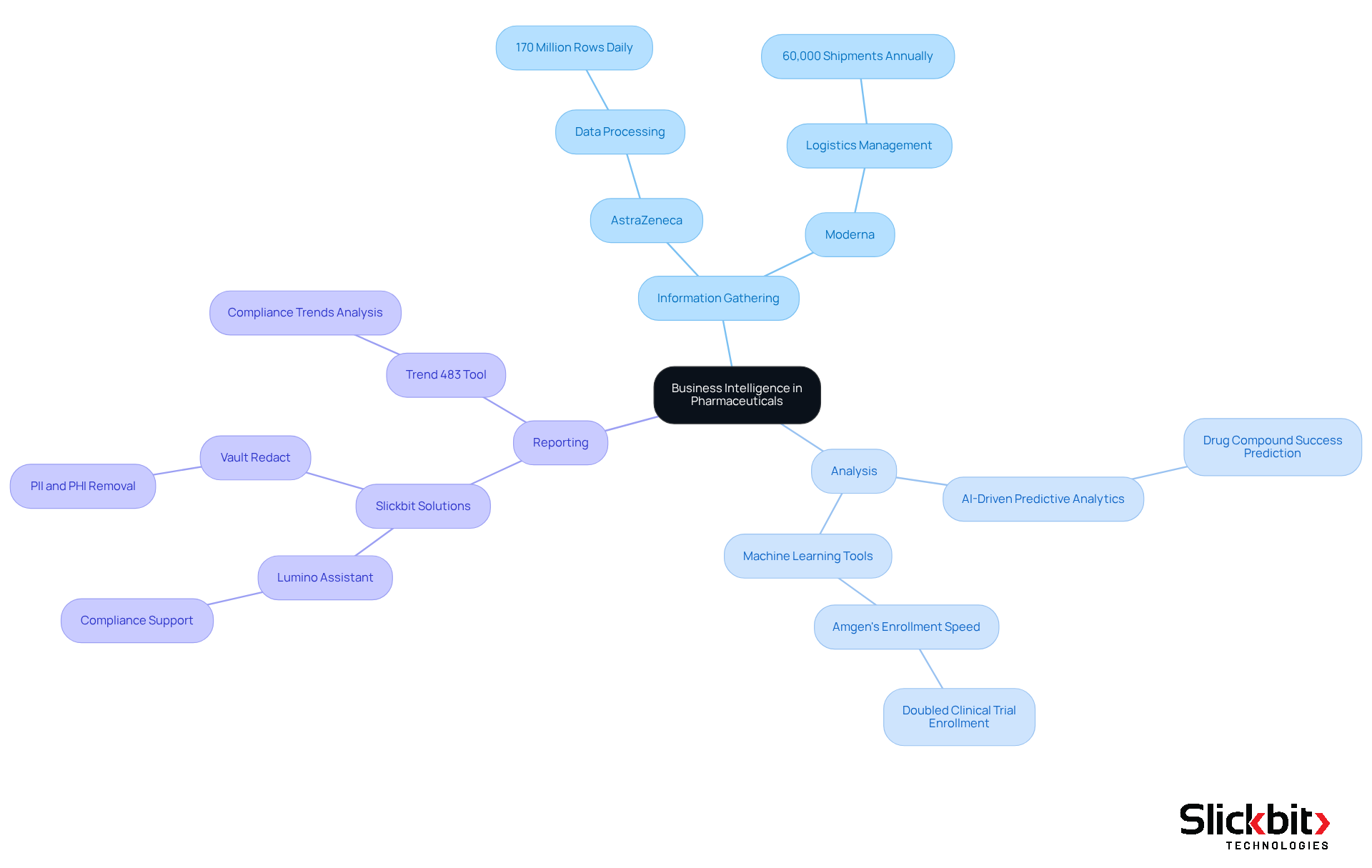
Business intelligence pharmaceutical includes a suite of technologies and strategies designed to analyze information, yielding actionable insights that enhance decision-making in research and development (R&D). In this critical sector, business intelligence pharmaceutical plays a pivotal role in streamlining processes, ensuring compliance, and enhancing the overall efficiency of drug development. Key components of BI include information gathering, analysis, and reporting, which collectively empower organizations to make informed decisions based on real-time insights.
The integration of business intelligence pharmaceutical with Artificial Intelligence (AI) and Machine Learning (ML) is revolutionizing research and development in the pharmaceutical sector. For instance, AstraZeneca's BI solution processes over 170 million rows of data daily, significantly boosting operational efficiency and decision-making capabilities. Furthermore, AI-driven predictive analytics can identify which drug compounds are most likely to succeed early in the development phase, conserving both time and resources.
Real-world applications of business intelligence pharmaceutical underscore its effectiveness. Moderna, for example, manages over 60,000 shipments annually through BI solutions, ensuring timely deliveries while minimizing waste via real-time visibility into logistics and inventory levels. Additionally, companies like Amgen have doubled their clinical trial enrollment speed by employing data-driven machine learning tools, showcasing how BI can enhance recruitment strategies.
Slickbit's AI-powered solutions further illustrate the potential of business intelligence pharmaceutical in the pharmaceutical industry. The Lumino assistant equips pharma teams with accurate, traceable answers sourced from FDA and global guidance documents, bolstering compliance efforts. Vault Redact automates the identification and removal of PII and PHI from documents, streamlining regulatory processes. The Trend 483 tool identifies trends in compliance, providing deeper insights into systemic risks and repeat violations. Moreover, the innovative AI Voice Agent for Shipment Alerts facilitates real-time shipment monitoring, contributing to operational efficiency by ensuring timely updates and effective logistics management.
As the healthcare landscape evolves, leveraging advanced business intelligence pharmaceutical technologies enables organizations to optimize resource allocation, identify trends, and accelerate the drug development timeline, ultimately facilitating faster patient access to new treatments. This strategic approach enhances adherence and equips companies to navigate the complexities of the pharmaceutical industry effectively. As Bill Coyle aptly noted, "The industry is experiencing an unprecedented time of data-driven scientific breakthroughs.
Explore Key Business Intelligence Tools for R&D
Several key business intelligence pharmaceutical tools significantly benefit R&D teams, each offering unique features that enhance data analysis and decision-making.
-
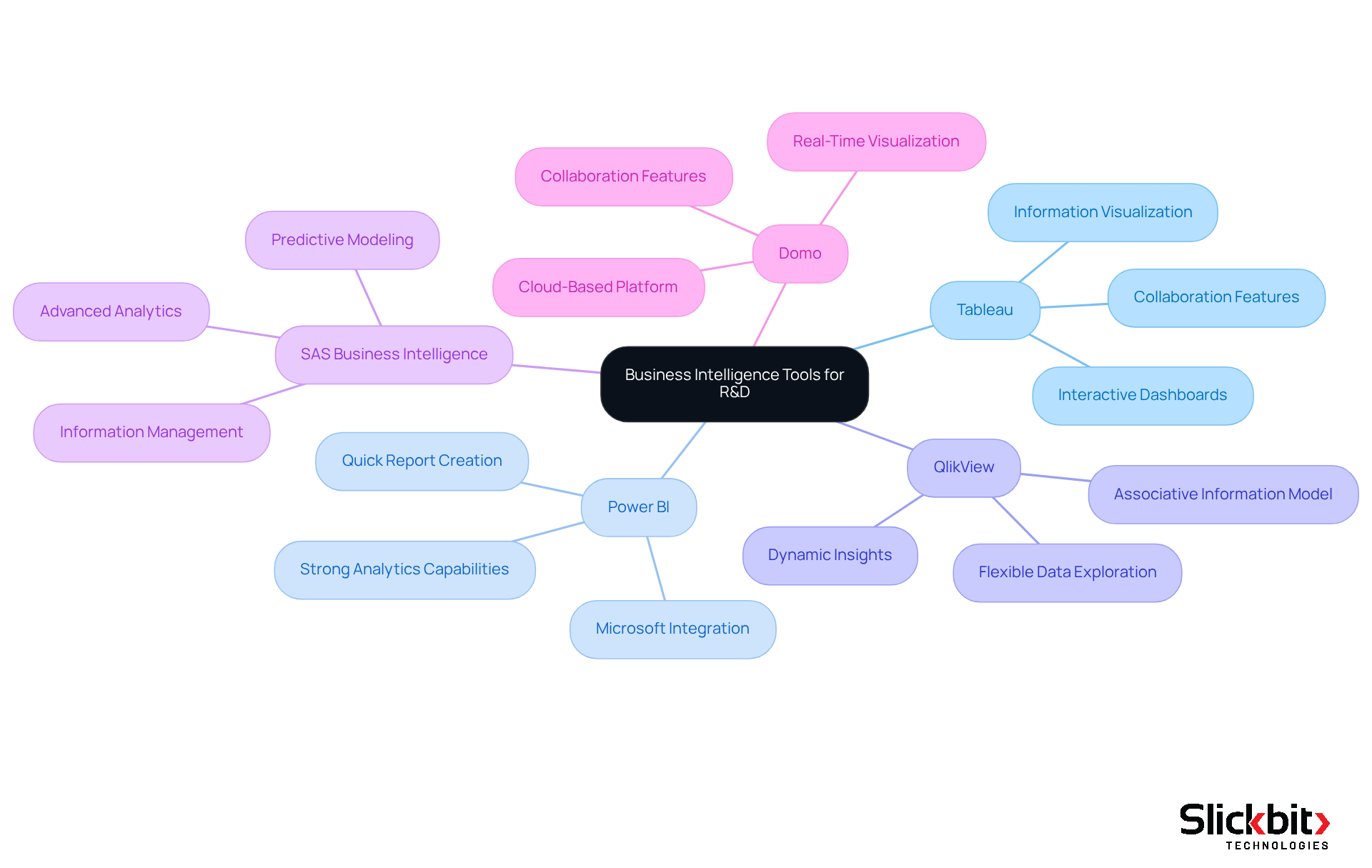
Tableau, renowned for its powerful information visualization capabilities, enables teams to create interactive and shareable dashboards. This functionality simplifies the analysis of complex datasets and facilitates collaboration across departments.
-
In addition, Power BI, developed by Microsoft, stands out for its seamless integration with other Microsoft products. It provides strong analytics capabilities, allowing users to create reports and dashboards quickly, which is essential for prompt decision-making in R&D.
-
Furthermore, QlikView, with its associative information model, empowers users to explore content freely, uncovering insights that may not be readily apparent. This flexibility is especially valuable in the dynamic setting of drug research.
-
SAS Business Intelligence pharmaceutical is recognized for its advanced analytics and predictive capabilities, making it well-suited for complex information analysis in the pharmaceutical sector. It aids in information management and provides resources for predictive modeling, crucial for drug development processes.
-
Lastly, Domo excels as a cloud-based platform, offering real-time information visualization and collaboration features. This makes it ideal for teams that need to share insights quickly and efficiently, enhancing responsiveness in R&D initiatives.
Selecting the appropriate resource depends on the specific requirements of the R&D team, including the types of data sources, user proficiency, and the capacity to integrate with current systems. The efficiency of these resources in enhancing operational productivity and data-informed decision-making is increasingly acknowledged in the business intelligence pharmaceutical industry.
Implement Business Intelligence Tools in R&D Workflows
To effectively implement business intelligence (BI) tools in R&D workflows, it is essential to follow these structured steps:
-
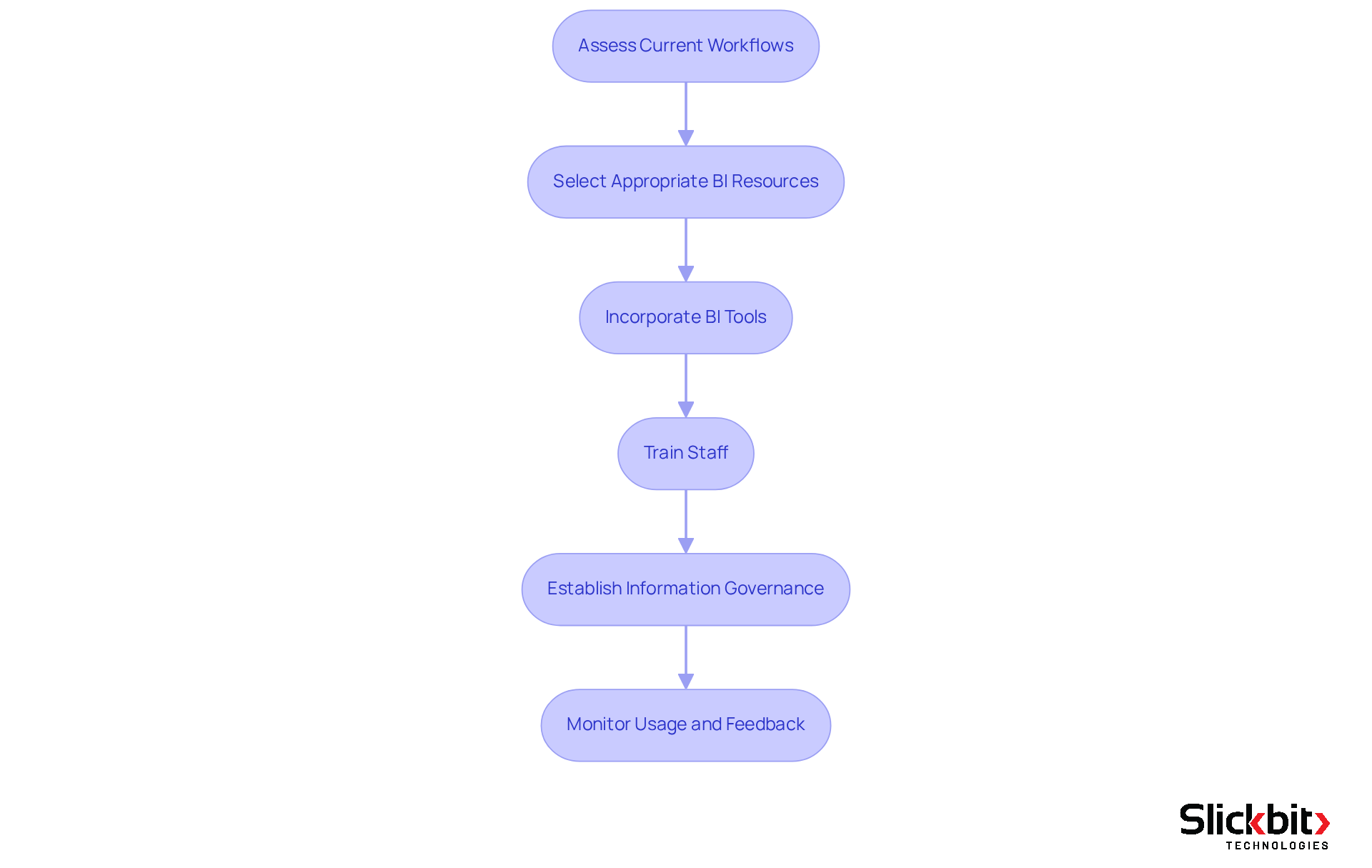
Assess Current Workflows: Begin by evaluating existing R&D processes to pinpoint areas where BI can enhance efficiency. Identify bottlenecks, information silos, and inefficiencies that obstruct productivity.
-
Select Appropriate BI Resources: Based on your evaluation, choose BI resources that align with the identified needs. Consider factors such as user-friendliness, integration capabilities with existing systems, and scalability to accommodate future growth.
-
Incorporate BI Tools: Collaborate with IT and information management teams to seamlessly incorporate the chosen BI tools into existing systems. Ensure smooth information flow between platforms to maintain integrity and enhance operational efficiency. It is crucial to guarantee compatibility with current pharmaceutical information systems, including business intelligence pharmaceutical systems, laboratory information management systems, and ERP.
-
Train Staff: Conduct comprehensive training sessions for R&D teams to familiarize them with the new tools. Highlight the significance of data-driven decision-making and demonstrate how to leverage BI insights for improved outcomes.
-
Establish Information Governance: Implement robust information governance policies to ensure quality, security, and compliance with regulatory standards. This is paramount in the business intelligence pharmaceutical industry, where adherence to regulations is critical. Emphasizing data quality and security is essential to mitigate risks associated with compliance.
-
Monitor Usage and Feedback: After implementation, continuously observe the usage of BI resources and collect feedback from users. This will assist in recognizing any obstacles and opportunities for enhancement, ensuring that the resources effectively satisfy the requirements of the R&D teams.
Statistics indicate that organizations integrating BI tools into their R&D workflows can experience significant improvements in efficiency and decision-making speed. For instance, organizations utilizing BI for information analysis have reported up to a 30% decrease in time dedicated to management tasks, enabling teams to concentrate more on innovation and strategic initiatives. Additionally, BI aids in the efficient management of clinical trials by integrating and reporting data in real time, allowing for faster adjustments and streamlined processes. By leveraging business intelligence pharmaceutical, drug companies can enhance their R&D capabilities, leading to faster drug development cycles and improved compliance with regulatory requirements.
Monitor and Evaluate Business Intelligence Impact on R&D
To effectively monitor and evaluate the impact of business intelligence (BI) on research and development (R&D) in pharmaceuticals, it is essential to follow these structured steps:
-
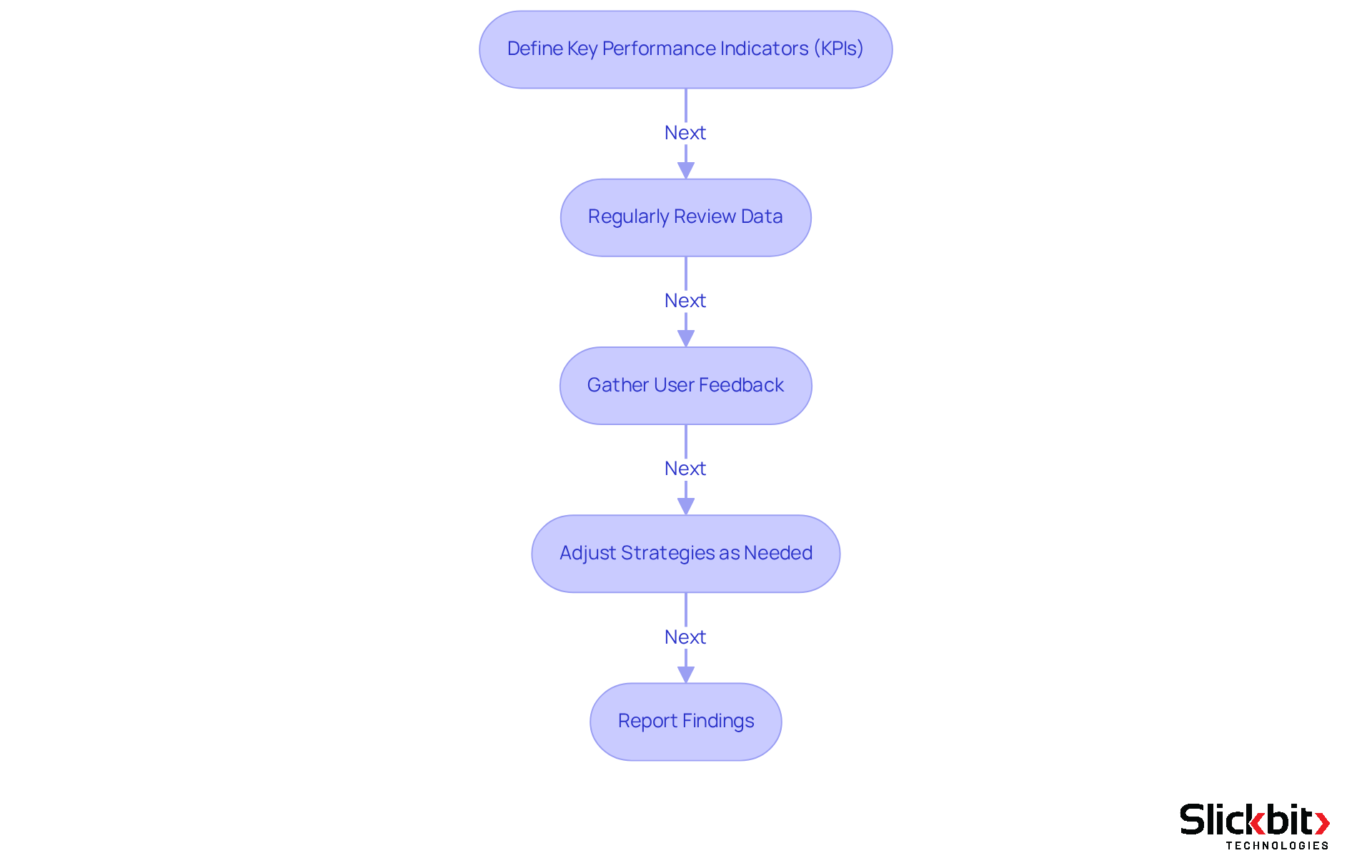
Define Key Performance Indicators (KPIs): Establish KPIs that align with your R&D objectives. Important metrics may include time-to-market statistics, compliance rates, clinical trial success rates, and resource utilization efficiency. For instance, monitoring the average duration from drug creation to market introduction can demonstrate the efficiency of BI resources in expediting development cycles. Key performance indicators for pharmaceutical companies, such as drug approval rates and clinical trial success, are critical for assessing the impact of business intelligence pharmaceutical.
-
Regularly Review Data: Arrange consistent evaluations of the data produced by BI systems. Examine trends and patterns to assess whether the resources are meeting the established KPIs. For example, monitoring the 'Average Requests Closing Time' can provide insights into how quickly maintenance tasks are resolved, reflecting overall operational efficiency. Regular reviews can help identify areas for improvement, as evidenced by case studies where companies optimized their supply chains through effective BI utilization.
-
Gather User Feedback: Conduct surveys or interviews with R&D staff to collect qualitative insights on the usability and effectiveness of the BI resources. This feedback can highlight areas needing improvement and inform future enhancements. Engaging users in this manner ensures that the BI implementation remains relevant and user-friendly. As noted by industry experts, user engagement is crucial for successful BI adoption.
-
Adjust Strategies as Needed: Based on data analysis and user feedback, make necessary adjustments to the BI implementation. This may involve improving processes, offering further training, or even changing resources if current solutions do not meet expectations. For instance, if a specific BI application is not providing the expected insights, exploring alternatives like Wyn Enterprise may be advantageous. The flexibility of BI tools allows organizations to adapt swiftly to changing needs.
-
Report Findings: Generate detailed reports outlining the effect of BI on R&D activities and distribute these to stakeholders. Highlight successes, such as improved compliance or reduced time-to-market, and identify areas for further development to ensure ongoing support for BI initiatives. Utilizing dashboards for real-time insights can enhance the clarity and accessibility of these reports. As KanBo emphasizes, effective reporting is essential for driving strategic decisions and ensuring alignment across departments.
By systematically implementing these steps, pharmaceutical companies can effectively leverage business intelligence to enhance their R&D capabilities, ultimately leading to faster and more compliant drug development processes.
Conclusion
The integration of business intelligence in pharmaceutical R&D represents a transformative approach to enhancing efficiency and decision-making. By harnessing advanced technologies and data analytics, organizations can streamline processes, improve compliance, and ultimately accelerate the drug development timeline. This strategic implementation not only facilitates faster access to new treatments for patients but also positions pharmaceutical companies to navigate the complexities of the industry with greater agility.
Throughout the article, key insights were shared regarding the role of business intelligence tools and their applications in real-world scenarios. Examples such as AstraZeneca's data processing capabilities and Moderna's logistics management illustrate the tangible benefits of BI solutions. Furthermore, the step-by-step guide for implementing these tools emphasizes the importance of:
- Assessing current workflows
- Selecting appropriate resources
- Establishing information governance to ensure successful integration
In conclusion, the significance of business intelligence in pharmaceutical R&D cannot be overstated. As the industry continues to evolve, embracing these technologies will be essential for organizations aiming to optimize their research capabilities and enhance patient outcomes. By taking action to implement BI tools effectively, pharmaceutical companies can not only drive innovation but also ensure they remain competitive in a rapidly changing landscape.
Frequently Asked Questions
What is business intelligence in pharmaceuticals?
Business intelligence in pharmaceuticals refers to a suite of technologies and strategies designed to analyze information, yielding actionable insights that enhance decision-making in research and development (R&D) within the pharmaceutical sector.
How does business intelligence improve drug development?
Business intelligence streamlines processes, ensures compliance, and enhances overall efficiency in drug development by enabling organizations to make informed decisions based on real-time insights from information gathering, analysis, and reporting.
What role do AI and ML play in business intelligence for pharmaceuticals?
AI and Machine Learning are integrated with business intelligence to revolutionize research and development by processing large volumes of data, identifying successful drug compounds early in development, and significantly boosting operational efficiency and decision-making capabilities.
Can you provide an example of a company using business intelligence effectively?
Moderna manages over 60,000 shipments annually through business intelligence solutions, ensuring timely deliveries and minimizing waste through real-time visibility into logistics and inventory levels.
How has business intelligence impacted clinical trial enrollment?
Companies like Amgen have doubled their clinical trial enrollment speed by employing data-driven machine learning tools, demonstrating how business intelligence can enhance recruitment strategies.
What are some specific tools mentioned that utilize business intelligence in pharmaceuticals?
Some tools include Slickbit's Lumino assistant for compliance, Vault Redact for automating the removal of PII and PHI from documents, the Trend 483 tool for identifying compliance trends, and an AI Voice Agent for real-time shipment alerts.
What are the benefits of leveraging advanced business intelligence technologies in healthcare?
Leveraging advanced business intelligence technologies allows organizations to optimize resource allocation, identify trends, accelerate the drug development timeline, and ultimately facilitate faster patient access to new treatments.




