Overview
The article addresses critical tools that enhance regulatory intelligence within the life sciences sector, underscoring the pivotal role of AI-driven solutions in bolstering compliance and operational efficiency. It presents a range of software and services, including Slickbit's AI tools and Veeva Vault, which offer customized solutions to navigate regulatory challenges. These tools not only streamline processes but also promote innovation in drug development, ultimately fostering a more agile and compliant industry. By leveraging such advanced technologies, organizations can significantly improve their regulatory strategies and drive forward the evolution of drug development.
Introduction
The landscape of regulatory compliance in the Life Sciences sector is undergoing a transformative shift, driven by the integration of advanced technologies and innovative solutions. As regulations become increasingly complex and the demand for efficiency escalates, organizations are compelled to harness tools that enhance their regulatory intelligence. This article explores eight key tools that not only streamline compliance processes but also empower companies to navigate the intricate regulatory environment with confidence.
How can these cutting-edge solutions reshape the future of regulatory oversight and ensure that organizations stay ahead in a competitive market?
Slickbit: AI Solutions for Streamlined Regulatory Compliance
Slickbit excels in providing customized AI solutions that significantly enhance regulatory intelligence for organizations in the Life Sciences sector. By rapidly creating enterprise-level Minimum Viable Products (MVPs) in just four weeks, Slickbit empowers companies to adopt AI-driven oversight intelligence tools, such as the AI-powered Oversight Intelligence assistant and Vault Redact. These tools greatly improve operational efficiency while ensuring compliance with rigorous standards. This focus on the unique challenges faced by R&D managers allows for tailored solutions that address specific compliance needs, ultimately boosting productivity and fostering innovation throughout the drug development lifecycle.
Recent advancements in AI indicate a potential reduction in drug discovery costs by up to 40%. Additionally, AI-driven tools streamline patient recruitment and enhance trial design, resulting in faster and more reliable outcomes. Industry leaders recognize that integrating AI into oversight processes improves regulatory intelligence, accelerates compliance, and transforms the drug development landscape. Consequently, it is imperative for organizations to embrace these innovations to maintain a competitive edge.
Furthermore, Slickbit's AI integration services and analytics capabilities further bolster operational efficiency, delivering valuable insights that support informed decision-making. The AI in pharma market was valued at $1.8 billion in 2023 and is projected to experience significant growth, underscoring the increasing importance of AI within the industry. As Bhavik Shah from McKinsey emphasizes, companies must understand and scale AI to unlock its full potential.
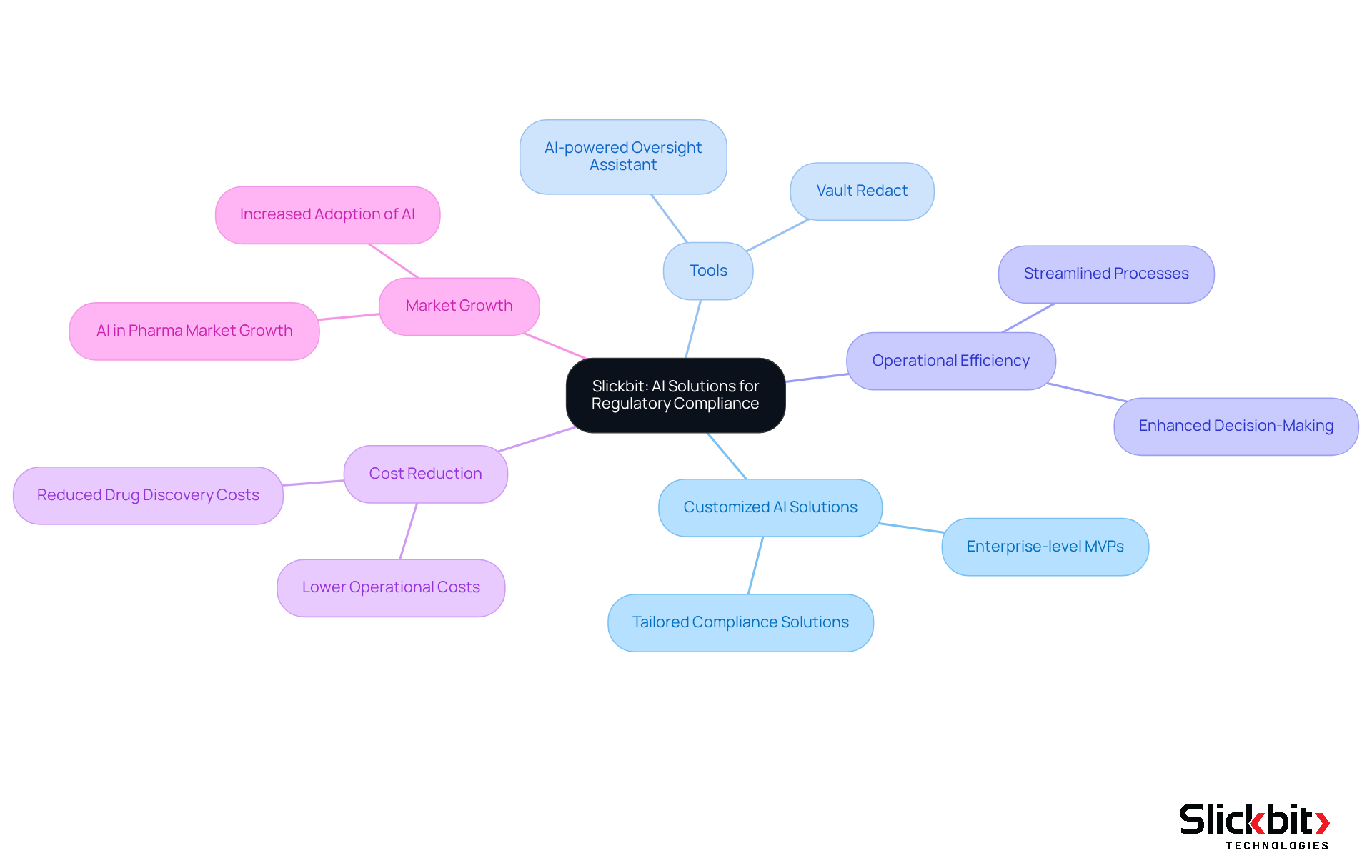
Veeva Vault: Comprehensive Regulatory Document Management
Veeva Vault offers a robust cloud-based solution for managing compliance documents, ensuring that all critical files are systematically organized and readily accessible. Its comprehensive features include:
- Version control
- Audit trails
- Collaboration tools
All designed to support adherence to legal requirements. By centralizing document management, Veeva Vault empowers Life Sciences companies to maintain precise records and enhance regulatory intelligence during the submission process to regulatory authorities.
In addition, Slickbit's AI-driven solutions, such as Vault Redact, automate the identification and removal of Personally Identifiable Information (PII) and Protected Health Information (PHI) from documents, thereby enhancing security and compliance with regulations. Furthermore, Trend 483 utilizes AI to detect trends in systemic risks and compliance issues derived from FDA 483s, providing deeper insights that complement the document management capabilities of Veeva Vault. Together, these innovations not only improve operational efficiency but also fortify organizations against regulatory intelligence challenges.
ArisGlobal: Advanced Software for Regulatory Intelligence
Slickbit's software solutions are essential for enhancing regulatory intelligence, providing robust data analytics and reporting capabilities that cover the entire lifecycle of submissions—from initial planning to post-market monitoring. By leveraging advanced analytics, including the AI-driven Lumino assistant, Slickbit empowers Life Sciences companies to make data-informed decisions, ensuring adaptability in an evolving compliance landscape.
As the pharmaceutical sector undergoes a significant shift towards data-driven methodologies, the demand for compliance intelligence software is on the rise. Organizations that effectively manage their submission lifecycle can achieve:
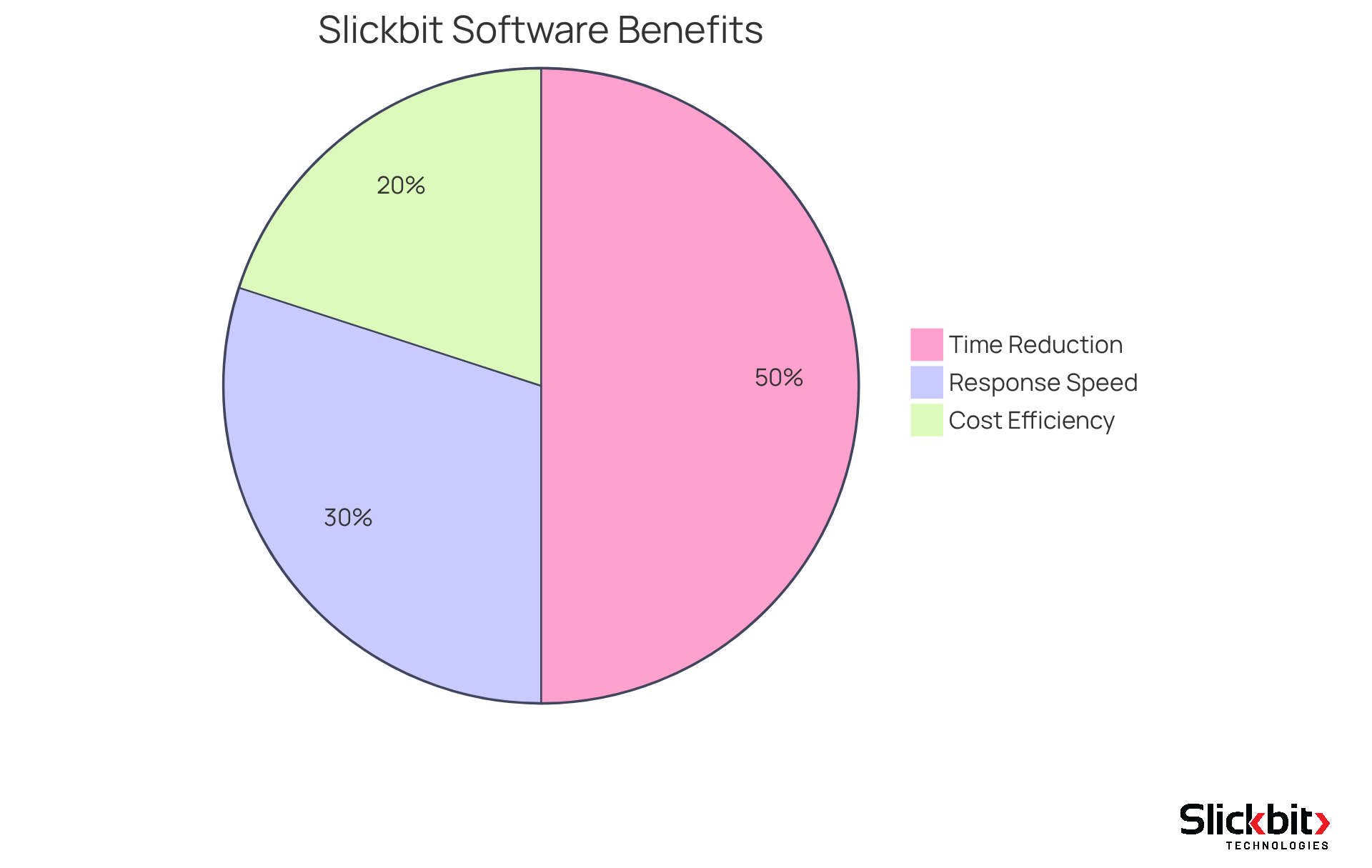
- Up to a 50% reduction in time to database lock
- A 20% improvement in cost efficiency
Moreover, the potential for $3 billion to $5 billion in efficiency gains within medical affairs highlights the critical significance of these solutions. With the market for oversight intelligence software projected to expand by at least 120% year-over-year, Slickbit distinguishes itself by offering customized solutions such as:
- Vault Redact for secure content redaction
- Trend 483 for adherence insights
These tools not only streamline processes but also enhance overall operational efficiency, positioning organizations for success in a competitive landscape. Additionally, utilizing Slickbit's software can result in a 30% faster response to Health Authority Queries (HAQs), further underscoring the efficiency benefits.
To fully leverage the advantages of regulatory intelligence, companies should integrate expert insights and best practices into their workflows using regulatory intelligence software.
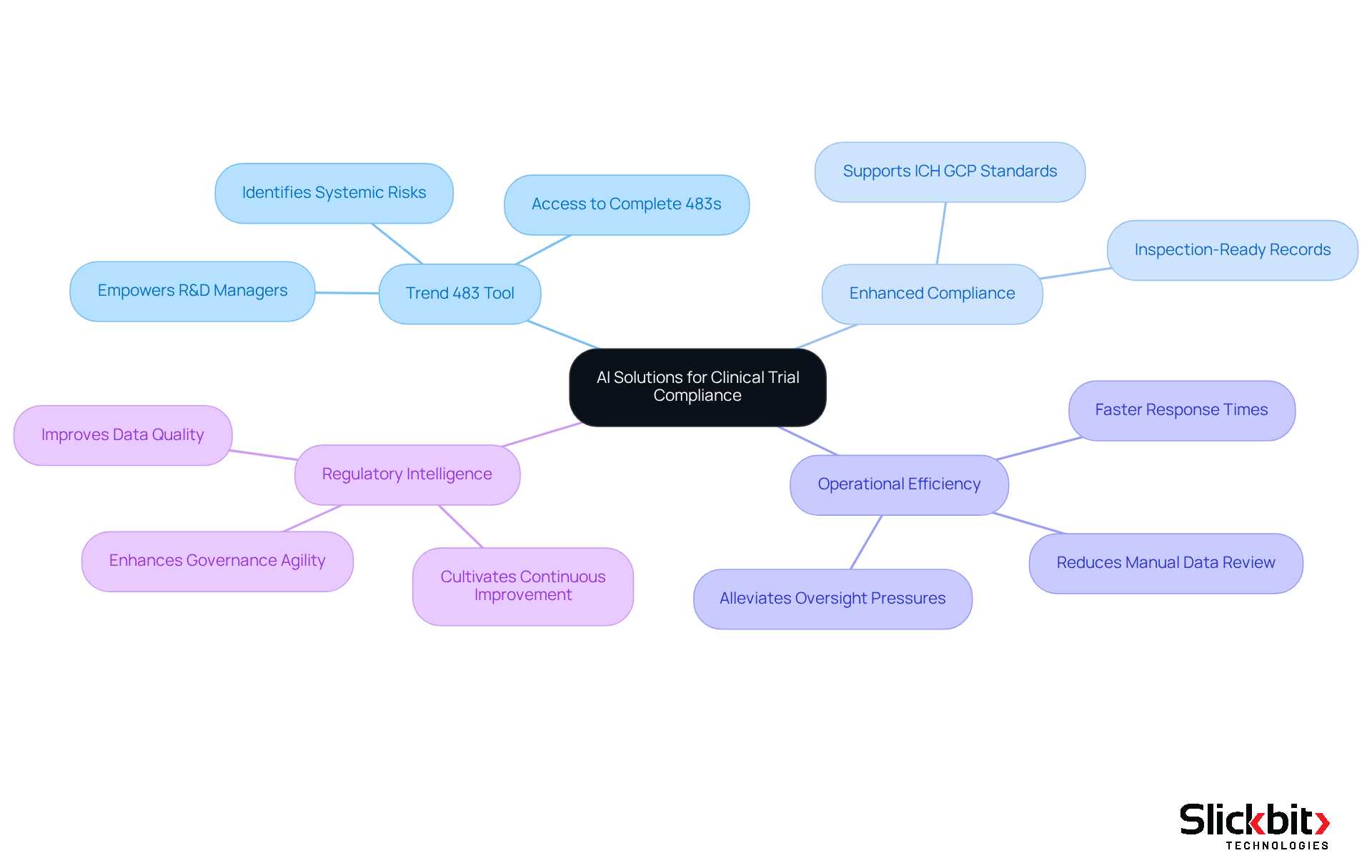
Medidata: Cloud Solutions for Clinical Trial Compliance
Slickbit offers a comprehensive suite of AI-driven solutions designed to enhance adherence and operational efficiency within pharmaceutical affairs. Their Trend 483 tool utilizes advanced AI technology to identify trends in systemic risks, repeated violations, and regulatory challenges stemming from FDA 483s. This functionality empowers pharmaceutical R&D managers to search, filter, and access complete 483s directly, providing profound insights into regulatory hurdles. By leveraging such innovative tools, Life Sciences companies can substantially improve their regulatory intelligence and optimize their operations.
Moreover, the integration of AI into regulatory intelligence processes not only enhances data quality but also enables teams to concentrate on high-value tasks, reducing the burden of manual data review. This transformation leads to increased efficiency and faster response times in addressing regulatory issues. Statistics indicate that employing AI tools like Trend 483 can alleviate pressures on oversight teams, ultimately fostering a more agile and responsive approach to governance challenges.
As organizations navigate the intricate landscape of FDA regulations, the insights derived from AI-driven solutions can bolster their regulatory intelligence, ensuring that standards are not merely met but exceeded, cultivating a culture of continuous improvement and operational excellence.
Oracle Siebel Life Sciences: Managing Regulatory Processes
Oracle Siebel Health Sciences provides a comprehensive suite of applications tailored for the effective management of compliance processes. Its functionalities encompass:
- Tracking submissions
- Managing compliance documentation
- Enhancing communication with governing bodies
By leveraging Oracle Siebel, Life Sciences organizations can enhance their regulatory intelligence to streamline compliance workflows, ensuring adherence to industry standards while improving overall efficiency. Notably, the system generates distinct and sequential report numbers, which are crucial for maintaining accurate records during compliance submissions.
Furthermore, it facilitates the synchronization of target properties between the Enterprise Manager (EM) and the actual Siebel configuration, thereby mitigating discrepancies that could hinder compliance. This capability is vital for managing documentation related to product complaints and adverse events, ensuring that reports are formatted correctly for submission to the FDA and aligned with regulatory intelligence requirements.
In conclusion, Oracle Siebel serves as an indispensable tool for businesses in the Sciences sector striving to enhance their oversight capabilities and uphold standards in a demanding environment.
ComplianceQuest: Quality Management for Regulatory Compliance
ComplianceQuest offers a robust cloud-based quality management system tailored for Life Sciences organizations, significantly enhancing regulatory intelligence. Its key features—document control, training management, and audit management—are essential for upholding high-quality standards. By leveraging ComplianceQuest, businesses can streamline their compliance processes, thereby minimizing the risk of legal infractions. As W. Edwards Deming wisely stated, 'Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution.' This highlights the necessity of a systematic approach to quality management, particularly in the current regulatory environment.
Current trends indicate a growing reliance on cloud-based quality management systems, which provide the flexibility and scalability required for organizations to adapt to evolving regulatory intelligence requirements. By embracing these systems, organizations within the health sciences sector can not only enhance their compliance efforts but also foster a culture of continuous improvement. This aligns with the principles of Total Quality Management, which advocate for proactive quality oversight and data-driven decision-making.
QAD Cloud ERP: Optimizing Operations for Compliance
QAD Cloud ERP serves as a robust solution for Life Sciences firms, specifically designed to enhance operations while emphasizing regulatory adherence. Its comprehensive features include:
- Supply chain management
- Production planning
- Quality control
Each is critical for meeting stringent regulatory standards. By leveraging QAD Cloud ERP alongside Slickbit's AI-driven tools such as Trend 483 and Vault Redact, organizations can significantly improve operational efficiency and effectively maintain compliance with industry regulations.
Trend 483 utilizes AI to identify patterns in systemic risks and adherence derived from FDA 483s, providing deeper insights that bolster the operational capabilities of QAD Cloud ERP. Vault Redact automates the identification and removal of PII and PHI from documents, further supporting regulatory efforts within the pharmaceutical sector.
Additionally, Lumino functions as an AI-powered assistant for regulatory intelligence, allowing pharma teams to obtain accurate, traceable answers from FDA and global guidance documents. This dual focus on optimization and regulation positions QAD Cloud ERP as an indispensable tool for navigating the complexities of the pharmaceutical landscape.
Actionable Tip: To enhance adherence, organizations should routinely evaluate their operational processes within QAD Cloud ERP, ensuring alignment with evolving legal standards while harnessing AI insights from Slickbit.
PharmaLex: Regulatory Affairs Consulting for Life Sciences
PharmaLex excels in providing regulatory intelligence consulting, delivering invaluable expertise to Life Sciences companies as they navigate the complexities of oversight environments. Their extensive services encompass strategy development, submission management, and compliance audits, all meticulously customized to address the unique needs of the pharmaceutical sector. By partnering with PharmaLex, organizations can significantly enhance their regulatory intelligence, thereby positioning themselves to effectively tackle challenges related to regulations.
Current trends indicate a growing emphasis on proactive governance strategies, particularly in response to the increasing complexity of drug regulations and the globalization of the pharmaceutical industry, which underscores the need for regulatory intelligence. For instance, the pharmaceutical compliance affairs market is projected to expand at a CAGR of 7.17% from 2025 to 2030, reaching USD 14.34 billion by 2030, driven by heightened oversight demands and a focus on patient safety.
Success narratives abound, illustrating how PharmaLex has empowered businesses in the Sciences sector to streamline their compliance procedures. By leveraging their expertise, organizations have achieved timely submissions and compliance, ultimately accelerating their time-to-market. Experts in regulatory intelligence emphasize that meticulous documentation and adherence to Good Manufacturing Practices are essential for navigating these intricate landscapes.
In conclusion, PharmaLex emerges as an indispensable partner for Life Sciences companies, equipping them with the tools and insights necessary to thrive in a dynamic compliance environment.
Celerion: Clinical Research Services for Compliance
Celerion provides clinical research services that are essential for ensuring compliance in drug development. Their expertise spans:
- Conducting clinical trials
- Bioanalytical testing
- Compliance submissions
By leveraging Celerion's services, Life Sciences firms can ensure that their clinical research initiatives meet compliance standards. This adherence not only facilitates regulatory intelligence but also significantly enhances the likelihood of successful product approvals.
Bioclinica: Imaging Services for Regulatory Compliance
Slickbit plays a pivotal role in ensuring adherence to regulations through its innovative AI-powered solutions tailored for the pharmaceutical sector, including Lumino, Vault Redact, and Trend 483.
- Lumino serves as an AI-driven assistant for regulatory intelligence, empowering pharma teams to access accurate, traceable answers from FDA and global guidance documents.
- Vault Redact automates the identification and removal of PII and PHI from documents, ensuring sensitive information is handled securely.
- Furthermore, Trend 483 leverages AI to identify patterns in systemic risks and adherence issues from FDA 483s, enabling teams to search, filter, and gain deeper insights into oversight challenges.
By harnessing Slickbit's expertise, Life Sciences firms can effectively manage their adherence data, ensuring compliance with industry standards and enhancing the success of submissions. Organizations that align their adherence processes with evolving regulatory frameworks are 30% more likely to achieve favorable project outcomes. This proactive approach not only bolsters compliance but also streamlines the overall clinical trial process, positioning companies for success in a competitive landscape.
Conclusion
The landscape of regulatory intelligence in the Life Sciences sector is evolving at a rapid pace, highlighting the urgent need for organizations to adopt advanced tools and technologies that enhance compliance and operational efficiency. This article underscores eight key tools, including Slickbit, Veeva Vault, and ComplianceQuest, each meticulously designed to navigate the complexities of regulatory requirements while fostering innovation and productivity. By leveraging these solutions, companies can meet stringent standards and secure a competitive edge in the marketplace.
Critical insights emerge regarding the integration of AI solutions, particularly those offered by Slickbit, which streamline processes and significantly reduce costs associated with drug discovery and compliance management. The comprehensive capabilities of tools like Veeva Vault and Oracle Siebel further illustrate the necessity of robust document and process management in maintaining regulatory adherence. Moreover, the role of consulting firms such as PharmaLex emphasizes the importance of strategic guidance in navigating the intricate regulatory landscape.
Ultimately, the significance of regulatory intelligence cannot be overstated. As the industry faces increasing scrutiny and complexity, organizations must embrace these innovative tools and practices to ensure compliance while driving continuous improvement and operational excellence. Adopting such technologies will empower Life Sciences companies to thrive in a dynamic environment, ultimately benefiting patient safety and advancing healthcare solutions.
Frequently Asked Questions
What is Slickbit and what services does it provide?
Slickbit specializes in customized AI solutions for regulatory compliance in the Life Sciences sector, focusing on enhancing regulatory intelligence and operational efficiency through tools like the AI-powered Oversight Intelligence assistant and Vault Redact.
How quickly can Slickbit create Minimum Viable Products (MVPs)?
Slickbit can rapidly create enterprise-level Minimum Viable Products (MVPs) in just four weeks.
What are the benefits of using AI-driven tools in regulatory compliance?
AI-driven tools improve operational efficiency, ensure compliance with rigorous standards, reduce drug discovery costs by up to 40%, streamline patient recruitment, and enhance trial design, leading to faster and more reliable outcomes.
What is Veeva Vault and what features does it offer?
Veeva Vault is a cloud-based solution for managing compliance documents, offering features such as version control, audit trails, and collaboration tools to support legal adherence and enhance regulatory intelligence.
How does Slickbit's Vault Redact enhance document security?
Vault Redact automates the identification and removal of Personally Identifiable Information (PII) and Protected Health Information (PHI) from documents, improving security and compliance with regulations.
What insights does Trend 483 provide?
Trend 483 utilizes AI to detect trends in systemic risks and compliance issues derived from FDA 483s, providing deeper insights that complement the document management capabilities of Veeva Vault.
How does Slickbit's software enhance regulatory intelligence?
Slickbit's software solutions provide robust data analytics and reporting capabilities that cover the entire lifecycle of submissions, enabling data-informed decision-making and adaptability in compliance.
What potential efficiency gains can organizations achieve by using Slickbit's solutions?
Organizations can achieve up to a 50% reduction in time to database lock, a 20% improvement in cost efficiency, and potential efficiency gains of $3 billion to $5 billion within medical affairs.
What is the market outlook for oversight intelligence software?
The market for oversight intelligence software is projected to expand by at least 120% year-over-year, highlighting the growing demand for compliance intelligence solutions.
How can companies maximize the benefits of regulatory intelligence?
Companies should integrate expert insights and best practices into their workflows using regulatory intelligence software to fully leverage its advantages.




