Overview
The article delineates the significant advantages of Manufacturing Execution Systems (MES) for R&D managers, underscoring improvements in operational efficiency, regulatory compliance, and cost savings. By illustrating how MES automates processes, facilitates real-time data access, and encourages collaboration, it is evident that these systems lead to enhanced productivity and quality within manufacturing environments. Furthermore, the integration of MES not only streamlines operations but also ensures adherence to regulatory standards, thereby reducing the risk of costly compliance issues. Consequently, R&D managers are empowered to make informed decisions that drive innovation and efficiency. In conclusion, adopting MES is not merely an operational upgrade; it represents a strategic move towards achieving excellence in manufacturing processes.
Introduction
The manufacturing landscape is evolving rapidly, driven by the need for efficiency, compliance, and innovation. As organizations strive to enhance operational performance, the implementation of Manufacturing Execution Systems (MES) emerges as a pivotal strategy for R&D managers. This article delves into the eight key benefits of MES, revealing how these systems not only streamline processes and improve decision-making but also foster collaboration and drive cost savings.
Furthermore, amidst the promise of these advancements, the question remains: how can R&D managers effectively leverage MES solutions to navigate the complexities of modern manufacturing while ensuring quality and compliance?
Slickbit: Accelerate Manufacturing Efficiency with AI-Driven MES Solutions
Slickbit.ai excels in developing AI-driven Manufacturing Execution Systems (MES full form in manufacturing) that empower R&D managers to significantly enhance operational efficiency. Our AI-driven Regulatory Intelligence assistant, Lumino, delivers precise, traceable responses from FDA and worldwide guidance documents, which are vital for ensuring adherence in pharmaceutical production. Furthermore, our Vault Redact solution automates the identification and removal of PII and PHI from documents, ensuring secure content redaction. By integrating these cutting-edge AI technologies, Slickbit accelerates production processes, enabling organizations to swiftly adapt to market demands while ensuring compliance with industry regulations. This innovative approach not only streamlines workflows but also equips companies to harness data-driven insights for ongoing improvement.
Industry leaders highlight that efficient solutions, specifically the MES full form in manufacturing, can result in significant improvements in productivity and quality, ultimately promoting operational excellence in the pharmaceutical sector. Successful implementation is essential as it establishes the groundwork for a robust and adaptable production environment, capable of addressing the evolving challenges of the industry. As Senthil Ranganathan, CEO, states, "Our partnership with Tech Mahindra merges profound production expertise with global scale to ignite genuine digital transformation." Together, we're facilitating intelligent production through scalable, AI-enabled solutions that represent the MES full form in manufacturing—driving Industry 4.0 adoption, operational excellence, and measurable business impact.
Manikantan NS, Global Head of Manufacturing Service Line, emphasizes the need for intelligence, agility, and sustainability in contemporary industry, highlighting the strategic importance of the MES full form in manufacturing for navigating these challenges. Additionally, our Trend 483 solution utilizes AI to detect patterns in systemic risks and compliance from FDA 483s, offering deeper insights into production processes.
Enhanced Operational Efficiency: Streamline Processes with MES
The MES full form in manufacturing refers to systems that significantly enhance production processes by automating routine tasks, thereby minimizing manual involvement. This automation not only accelerates production cycles but also reduces errors and optimizes resource allocation. Furthermore, by utilizing AI-driven analytics, organizations can effectively identify bottlenecks and streamline workflows, resulting in substantial productivity gains. As noted by Eric Kimberling, the MES full form in manufacturing provides real-time visibility into inventory levels, which is crucial for maintaining operational efficiency.
In addition, the incorporation of the MES full form in manufacturing has been demonstrated to shorten cycle times and boost throughput, making it an essential instrument for contemporary production settings. For instance, in the biotech sector, MES has automated critical processes such as batch tracking and compliance reporting, ensuring adherence to regulatory standards while enhancing overall efficiency. This strategic approach not only increases productivity but also empowers R&D managers to navigate the complexities of production in a rapidly evolving industry. Consequently, embracing the MES full form in manufacturing is not merely a technological upgrade; it is a vital step towards achieving operational excellence.
Real-Time Data Access: Improve Decision-Making with MES Insights
MES solutions empower R&D managers by providing real-time access to essential production data, which facilitates swift and informed decision-making. Furthermore, with AI-driven analytics, such as those offered by Slickbit's Trend 483 tool, organizations can continuously monitor production metrics, track performance, and proactively address issues. This capability not only enhances operational agility but also ensures that manufacturing processes align consistently with strategic objectives. By utilizing real-time insights from AI solutions, including Lumino for regulatory intelligence and Vault Redact for adherence, R&D managers can significantly enhance workflows, minimize downtime, and ultimately foster innovation in drug development.
Regulatory Compliance: Maintain Standards with MES Implementation
The MES full form in manufacturing, which refers to Manufacturing Execution Systems, is essential for organizations committed to upholding regulatory standards in the life sciences sector. By automating documentation processes, MES solutions, whose full form in manufacturing is manufacturing execution systems, ensure that all manufacturing activities comply with stringent industry regulations. Integrated verification mechanisms and robust reporting functionalities empower R&D managers to effortlessly oversee adherence to regulations, significantly mitigating the risk of rule violations and their potential repercussions.
For instance, a global pharmaceutical company that adopted MES for large-scale vaccine manufacturing reported enhanced oversight tracking, which expedited regulatory approvals. Similarly, a biotech firm utilizing MES for gene and cell therapy production improved traceability and minimized deviations, demonstrating how automated documentation can streamline regulatory compliance.
Moreover, MES generates real-time reports on key performance indicators (KPIs), such as yield rates and cycle times, which are vital for maintaining Good Manufacturing Practices (GMP). This capability not only boosts operational efficiency but also guarantees that every production step is accurately documented, facilitating smoother audits and inspections.
As regulatory landscapes evolve, the role of the MES full form in manufacturing in automating documentation for compliance becomes increasingly critical. By digitizing production records and enforcing adherence to standard operating procedures (SOPs), MES reduces human error and strengthens product integrity, ultimately aiding pharmaceutical manufacturers in effectively meeting their regulatory responsibilities.
Resource Management: Optimize Asset Utilization with MES
The MES full form in manufacturing refers to solutions that empower R&D managers to significantly enhance asset utilization by providing comprehensive visibility into resource availability and performance. By conducting meticulous data analysis of equipment usage and maintenance schedules, organizations can make informed strategic decisions regarding resource allocation. This approach ensures that assets are utilized efficiently, minimizing downtime and maximizing productivity. Notably, companies that have implemented the MES full form in manufacturing have reported substantial improvements in operational efficiency, achieving increases in asset utilization rates of up to 30%.
Furthermore, by integrating MES with Slickbit.ai's AI-driven solutions, such as Vault Redact for secure content filtering and Trend 483 for regulatory insights, organizations can discern patterns in resource performance. This facilitates proactive maintenance and optimized scheduling that align with production demands. Such a data-focused strategy not only simplifies operations but also supports adherence to regulatory standards, establishing the MES full form in manufacturing as an essential resource for Life Sciences firms aiming to enhance their production processes.
System Integration: Connect MES with Existing Technologies for Seamless Operations
Integrating the MES full form in manufacturing with existing technologies, such as ERP and quality management systems, is essential for achieving seamless operations throughout the manufacturing process. This connectivity facilitates smooth data flow between systems, empowering R&D managers to obtain a comprehensive view of production activities. Consequently, they can make informed, data-driven choices that significantly improve operational efficiency. The integration of MES with ERP systems not only streamlines workflows but also optimizes resource management, leading to improved productivity and reduced operational costs. For instance, organizations that successfully implement MES integration often report enhanced visibility into production metrics, enabling proactive adjustments and minimizing downtime.
Based on industry insights, the global production execution system (MES), known as the mes full form in manufacturing, is expected to reach $41.78 billion US by 2032, highlighting the importance of MES in contemporary production. This holistic approach to production operations is increasingly acknowledged as a crucial element for sustaining competitiveness in the swiftly changing pharmaceutical landscape. However, challenges such as data fragmentation and equipment compatibility can complicate the integration process. Therefore, careful planning and stakeholder involvement are necessary to navigate these complexities effectively.
Scalability: Adapt MES Solutions to Meet Growing Business Needs
The MES full form in manufacturing signifies that these solutions are inherently scalable, empowering organizations to respond adeptly to evolving business needs and market demands. R&D managers have the option to implement the MES full form in manufacturing in phases, thereby gradually enhancing its capabilities in alignment with organizational growth. This strategic flexibility guarantees that production processes remain both efficient and effective, irrespective of scale. Consequently, organizations can optimize their operations while maintaining a robust response to market fluctuations.
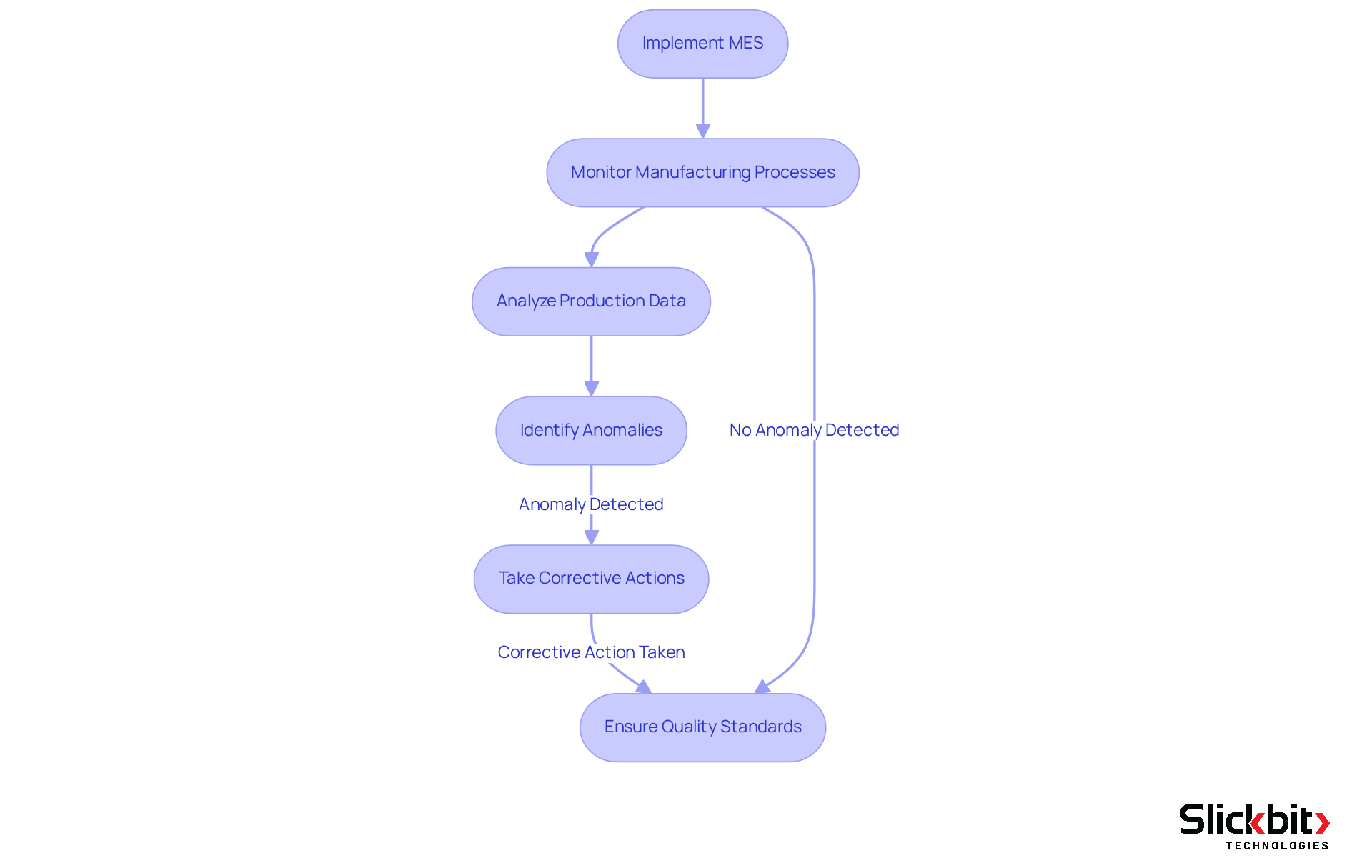
Quality Improvement: Elevate Product Standards with MES
Implementing the MES full form in manufacturing solutions significantly enhances product quality by providing advanced monitoring and control over manufacturing processes. This capability addresses the critical challenge of maintaining high standards in production. AI-driven analytics empower R&D managers to detect quality issues at an early stage, facilitating timely corrective actions that ensure products consistently meet the highest standards. Consequently, this proactive approach not only boosts customer satisfaction but also fortifies the organization's reputation in the competitive market.
For instance, AI-powered systems can analyze production data in real-time, identifying anomalies that may indicate potential defects. Such capabilities are essential for upholding adherence to strict regulatory standards, ultimately resulting in enhanced efficiency and product excellence. As industries increasingly adopt AI technologies, the synergy between advanced analytics and quality management is setting new benchmarks for product standards, driving innovation and competitiveness across the sector.
Cost Savings: Reduce Operational Expenses with MES Efficiency
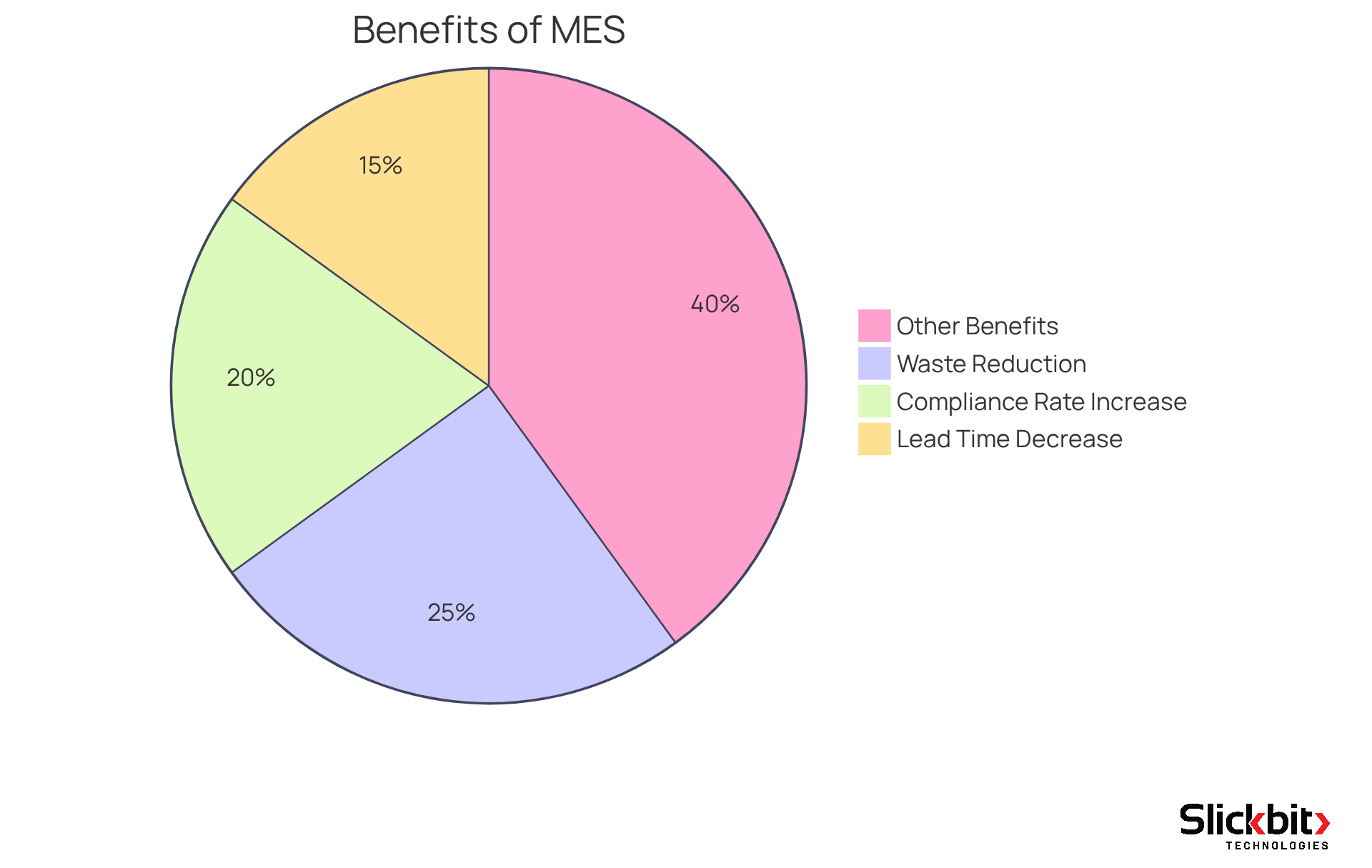
The MES full form in manufacturing indicates that implementing Manufacturing Execution Systems presents a compelling opportunity for organizations to achieve substantial cost savings by effectively minimizing expenses associated with inefficiencies and waste. By streamlining processes and optimizing resource utilization, R&D managers can significantly lower production costs while maintaining high-quality standards. For instance, companies that have adopted MES report waste reductions of up to 25%, according to Aberdeen Group, which directly enhances their bottom line. Furthermore, General Electric has documented a 15% decrease in lead times following MES implementation, as noted by McKinsey & Company, illustrating how enhanced efficiency translates into financial benefits.
The financial advantages of MES empower organizations to reinvest in innovation and growth, cultivating a culture of continuous improvement. As highlighted by industry leaders, effective change management is vital; organizations with robust strategies are six times more likely to meet or exceed project objectives, as per Prosci research. Additionally, PwC emphasizes that compliance rates can increase by 20% with MES, further improving efficiency and reducing potential costs related to regulatory compliance.
In the biotech sector, the impact of MES implementation has been transformative. For example, Comet's facility in Penang, Malaysia, successfully doubled its production volume within six months of adopting MES, which streamlined data collection and enhanced traceability. This not only bolstered efficiency but also facilitated improved financial planning and resource allocation, ultimately yielding significant cost savings.
Ultimately, the integration of solutions related to MES full form in manufacturing enables organizations to reduce costs, enhance decision-making processes, and maintain a competitive edge in the market.
Collaboration Enhancement: Foster Teamwork with MES Solutions
Slickbit's AI solutions significantly enhance collaboration among teams by providing a centralized platform for communication and data sharing. R&D managers play a crucial role in facilitating teamwork by ensuring that all stakeholders have access to real-time information, which enables them to work together more effectively. Furthermore, with Slickbit's rapid AI MVP development and custom solutions, teams can swiftly validate their ideas and implement intelligent AI agents that automate tasks and retrieve knowledge. This collaborative environment not only fosters innovation but also drives continuous improvement, highlighting the importance of understanding the mes full form in manufacturing. Consequently, it ultimately transforms business operations by enhancing regulatory intelligence and operational efficiency.
Conclusion
The implementation of Manufacturing Execution Systems (MES) signifies a transformative leap for R&D managers within the manufacturing sector, particularly in enhancing operational efficiency and compliance. By automating processes and leveraging AI-driven insights, organizations can streamline workflows, optimize resource utilization, and ultimately foster a culture of continuous improvement. The full form of MES in manufacturing encapsulates a comprehensive solution that addresses the evolving challenges faced by modern production environments.
Key benefits of MES are highlighted throughout the article, including:
- Enhanced operational efficiency
- Real-time data access
- Regulatory compliance
- Improved resource management
The integration of MES with existing technologies not only facilitates seamless operations but also supports scalability, allowing organizations to adapt to changing market demands. Furthermore, the emphasis on quality improvement and cost savings underscores the critical role that MES plays in driving productivity and maintaining high standards in the competitive landscape of manufacturing.
In conclusion, embracing MES solutions is not merely a technological upgrade but a strategic necessity for organizations aiming to thrive in the rapidly evolving manufacturing landscape. By prioritizing the implementation of MES, R&D managers can unlock significant operational benefits, enhance collaboration, and ultimately position their organizations for sustained success. It is crucial for industry leaders to recognize the importance of these systems and invest in their integration to remain competitive and responsive to market dynamics.
Frequently Asked Questions
What is Slickbit and what does it offer?
Slickbit specializes in developing AI-driven Manufacturing Execution Systems (MES) that enhance operational efficiency for R&D managers, particularly in the pharmaceutical sector.
What is the purpose of the AI-driven Regulatory Intelligence assistant, Lumino?
Lumino provides precise and traceable responses from FDA and worldwide guidance documents, which are crucial for ensuring compliance in pharmaceutical production.
How does the Vault Redact solution function?
Vault Redact automates the identification and removal of Personally Identifiable Information (PII) and Protected Health Information (PHI) from documents, ensuring secure content redaction.
What are the benefits of integrating AI technologies in manufacturing processes?
Integrating AI technologies accelerates production processes, enables organizations to adapt swiftly to market demands, ensures compliance with regulations, streamlines workflows, and harnesses data-driven insights for ongoing improvement.
How do MES solutions impact productivity and quality in manufacturing?
MES solutions automate routine tasks, minimize manual involvement, provide real-time visibility into inventory levels, shorten cycle times, and boost throughput, ultimately leading to significant improvements in productivity and quality.
What role does AI-driven analytics play in MES?
AI-driven analytics help organizations identify bottlenecks, streamline workflows, and continuously monitor production metrics, enhancing operational agility and informed decision-making.
What is the significance of real-time data access in MES?
Real-time data access empowers R&D managers to make swift and informed decisions, align manufacturing processes with strategic objectives, and proactively address issues, thereby enhancing overall efficiency.
How does Slickbit's Trend 483 solution contribute to compliance?
Trend 483 utilizes AI to detect patterns in systemic risks and compliance from FDA 483s, offering deeper insights into production processes and aiding in adherence to regulatory standards.
What is the overall goal of implementing MES in manufacturing?
The implementation of MES is aimed at achieving operational excellence by automating processes, enhancing productivity, and ensuring compliance with industry regulations, particularly in rapidly evolving sectors like pharmaceuticals.




