Overview
This article outlines essential strategies that Contract Development and Manufacturing Organizations (CDMOs) can implement to optimize pharmaceutical development. By leveraging innovative technologies, CDMOs can enhance their operational efficiency and accelerate drug development timelines. Furthermore, effective project management plays a crucial role in navigating the complexities of the pharmaceutical landscape, ensuring that projects remain on track and within budget. Data-driven decision-making is also emphasized, as it fosters compliance with regulatory standards and enhances overall project outcomes. Collectively, these strategies not only improve efficiency but also position CDMOs as leaders in the competitive pharmaceutical sector.
Introduction
The pharmaceutical industry is undergoing a significant transformation, driven by the imperative for expedited drug development and enhanced operational efficiency. Contract Development and Manufacturing Organizations (CDMOs) are pivotal in this evolution, providing innovative strategies that streamline processes and foster collaboration.
This article explores seven key strategies that CDMOs can implement to not only enhance pharmaceutical development but also maintain a competitive edge in a rapidly changing market.
How can these organizations leverage advanced technologies and collaborative practices to navigate the challenges of compliance, scalability, and quality control? The answers are encapsulated within the strategies discussed here, each meticulously designed to propel the industry forward.
Slickbit: Accelerate Drug Development with Rapid MVP Solutions
Slickbit.ai stands at the forefront of delivering Minimum Viable Products (MVPs) tailored for Life Sciences companies, empowering CDMOs to significantly accelerate their drug development timelines. By concentrating on swift MVP creation, Slickbit enables organizations to rapidly test innovative concepts while ensuring compliance with industry standards, effectively reducing time-to-market. This strategy not only enhances operational efficiency but also cultivates a culture of innovation within CDMO collaborations, allowing for a responsive approach to evolving market demands and compliance frameworks.
Furthermore, the integration of advanced technologies has been demonstrated to reduce MVP development time by approximately 35%, equipping companies to respond more dynamically to industry challenges. Successful implementations of MVP strategies have resulted in substantial revenue growth; for instance, some startups have seen their annual revenue soar from $1 million to $20 million within just two years. As the pharmaceutical landscape continues to evolve, the capacity of CDMOs to leverage rapid MVP solutions will be crucial for enhancing their competitive edge and driving successful drug development outcomes.
AI-Driven Regulatory Compliance: Enhancing CDMO Efficiency
AI technologies are revolutionizing compliance management within CDMO operations. By automating compliance monitoring and documentation, these organizations can significantly mitigate non-compliance risks and enhance operational efficiency.
For instance, Slickbit.ai's AI-powered solutions, such as Vault Redact, streamline the identification and removal of Personally Identifiable Information (PII) and Protected Health Information (PHI) from documents, ensuring secure content management.
Furthermore, Trend 483 employs AI to identify trends in systemic risks and compliance from FDA 483s, which enables the CDMO to analyze extensive regulatory data effectively. This proactive approach not only mitigates risks but also improves the overall quality of the drug development process.
For pharmaceutical R&D managers, leveraging these AI solutions is essential for staying abreast of the latest requirements and responding swiftly to changes. Consequently, to maximize adherence and operational efficiency, consider incorporating Slickbit's AI tools into your operations within the CDMO.
Collaborative Project Management: Optimizing Resource Allocation in CDMO Partnerships
Effective collaborative project management is crucial for optimizing resource allocation in CDMO partnerships. By establishing clear communication channels and shared objectives, contract development and manufacturing organizations can allocate resources efficiently, thereby reducing waste and enhancing productivity. The integration of project management tools, such as ProofHub and other task management software, facilitates real-time data sharing and task tracking, significantly enhancing collaboration between drug companies and their CDMO partners. This synergy not only leads to more successful project outcomes but also accelerates the time-to-market for new drugs.
Organizations that implement project management best practices—such as maintaining portfolio lists and facilitating project approval processes—are 2.5 times more successful, underscoring the critical role of structured approaches in achieving project goals. As Peter Drucker aptly noted, 'Time is the scarcest resource and unless it is managed nothing else can be managed.' Additionally, Benjamin Franklin emphasized that 'By failing to prepare, you are preparing to fail,' highlighting the necessity of effective resource management and planning in the pharmaceutical landscape.
Furthermore, the importance of laboratory capabilities and transparency in partnerships with a CDMO cannot be overlooked, as these elements are vital for ensuring compliance and efficiency.
Data-Driven Decision Making: Leveraging Analytics in CDMO Collaborations
Data-driven decision-making is critical for CDMO organizations that aim to optimize operational efficiency and enhance drug development outcomes. By harnessing advanced analytics, these organizations can extract valuable insights across the drug development spectrum, from resource allocation to market dynamics. This analytical framework empowers CDMO to make informed decisions that are aligned with their strategic objectives, resulting in improved project outcomes and accelerated time-to-market.
Furthermore, the implementation of data analytics tools enhances forecasting abilities and risk management, allowing these organizations to remain adaptable in a constantly evolving environment. As the global life science analytics market is projected to grow significantly—from approximately $11.97 billion in 2025 to an estimated $24.85 billion by 2034—the integration of analytics into CDMO partnerships is not merely beneficial but essential for maintaining a competitive edge.
As one expert noted, 'Data is the sword of the 21st century; those who wield it well, the samurai.' To effectively leverage these insights, contract development and manufacturing organizations should consider investing in robust analytics platforms that enable real-time data access and analysis.
Scalability Solutions: How CDMOs Support Pharmaceutical Growth
CDMO, or Contract Development and Manufacturing Organizations, are essential collaborators in driving industry growth by providing scalable and adaptable manufacturing solutions. Their capability to swiftly adjust production volumes enables pharmaceutical companies to effectively respond to fluctuating market demands without incurring substantial capital expenditures. This adaptability proves crucial in an environment characterized by rapidly changing drug pipelines and stringent compliance frameworks.
Industry leaders assert that partnering with a CDMO transcends mere outsourcing; it involves forming strategic alliances that leverage extensive scientific and regulatory expertise. For instance, 53Biologics underscores that their comprehensive support—from preclinical development to Good Manufacturing Practice (GMP) manufacturing—expedites the market introduction of innovative products.
Furthermore, the integration of advanced technologies, such as AI-driven modeling and automation, enhances the scalability of contract development and manufacturing operations, allowing them to maintain a competitive edge in the evolving healthcare landscape. According to the Massachusetts Institute of Technology’s Center for Biomedical Innovation, AI-driven modeling can significantly reduce process development time and improve batch consistency, reinforcing the technological advancements that enhance CDMO capabilities.
As the demand for specialized production capabilities increases, CDMOs are increasingly recognized as vital facilitators of medical innovation and efficiency. However, it is crucial to acknowledge that reliance on these organizations also introduces potential supply chain risks, which pharmaceutical companies must navigate with care.
The U.S. drug market has witnessed a rise in the utilization of CDMOs, highlighting their indispensable role in the industry due to the growing complexity of drug development. With a projected growth rate of 6.49% from 2025 to 2030, the importance of CDMOs in the pharmaceutical landscape is poised for significant expansion.
Innovative Technologies: Driving Competitive Advantage in CDMO Partnerships
Innovative technologies are fundamentally transforming CDMO relationships, offering a significant competitive edge to those who embrace them. By implementing advanced manufacturing techniques such as continuous manufacturing and digital twins, CDMO can significantly enhance operational efficiency while simultaneously reducing costs. Furthermore, the incorporation of AI-powered analytics, like Slickbit.ai's Regulatory Intelligence and Trend 483 tool, enables these organizations to refine their processes, enhance quality control, and improve decision-making capabilities. These AI solutions provide accurate, traceable answers from FDA guidance documents and identify trends in compliance, which are crucial for maintaining regulatory standards. This technological adoption not only positions CDMOs as industry leaders but also strengthens the collaborations of CDMOs with pharmaceutical companies eager to drive innovation. With the contract development and manufacturing organization market expected to expand from USD 238.92 billion in 2024 to USD 465.24 billion by 2032, the focus on advanced technologies will be essential for preserving a competitive edge in this changing environment.
Supply Chain Management: Ensuring Timely Deliveries in CDMO Operations
Effective supply chain management is essential for ensuring timely deliveries in CDMO processes. By adopting robust logistics strategies—such as optimizing inventory management and reducing lead times—contract development and manufacturing organizations can significantly enhance their supply chain efficiency. For instance, the integration of advanced technologies like real-time tracking systems allows for improved visibility and responsiveness, enabling companies operating as a CDMO to swiftly adapt to fluctuations in demand. This agility is crucial; a genuinely nimble contract development and manufacturing organization can scale operations from 5 tons to 15 tons within weeks, highlighting the importance of operational flexibility.
Furthermore, a well-managed supply chain not only boosts operational efficiency but also elevates customer satisfaction by ensuring that companies receive their products punctually. This is particularly vital in light of the ongoing challenges in the industry, where over 250 essential medications are currently in short supply in the United States. As industry expert Davuluri Sucheth Rao states, "A proactive contract development and manufacturing organization nurtures strong relationships with suppliers, engaging in open communication to build trust and mitigate risks and disruptions."
Ultimately, the strategic application of logistics and technology in supply chain management facilitates quicker drug development timelines, ensuring that CDMOs can meet the rigorous demands of the pharmaceutical sector while maintaining compliance with regulatory requirements. To enhance your operations, consider implementing a cloud-based procure-to-pay platform to improve supplier engagement and streamline your logistics processes.
Quality Control Practices: Maintaining Standards in CDMO Partnerships
Quality control practices are not merely beneficial but essential in CDMO partnerships, as they ensure that products meet the highest standards of safety and efficacy in the context of CDMO. To achieve this critical objective, CDMOs must implement rigorous quality assurance protocols that include:
- Regular audits
- Comprehensive testing
- Stringent compliance checks
A steadfast commitment to fostering a culture of quality within their operations not only builds trust with industry partners but also guarantees the consistent reliability of products. This dedication to quality meets regulatory requirements and enhances the reputation of the CDMO and its partners in the competitive marketplace.
Establishments that adopt advanced quality management practices, such as proactive risk management and effective corrective and preventive actions (CAPA), have demonstrated significant improvements in their delivery performance metrics. Furthermore, the FDA's Quality Management Maturity (QMM) program underscores the importance of leadership dedication to quality, which is vital for maintaining high standards and ensuring operational excellence in drug manufacturing. Consequently, prioritizing quality control is not just a regulatory obligation; it is a strategic imperative that can lead to enhanced operational performance and market competitiveness.
Future Trends: Preparing for the Next Generation of CDMO Services
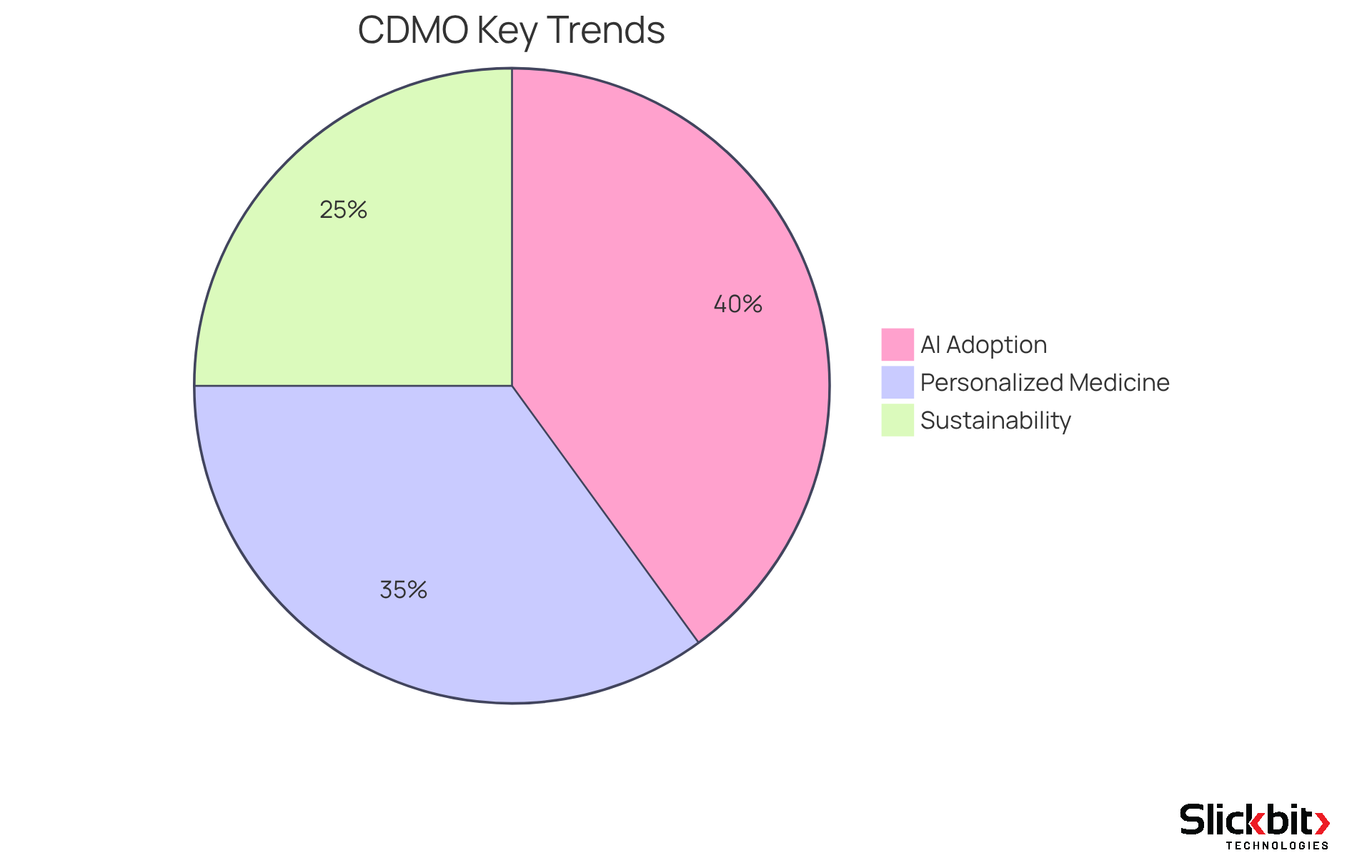
As the healthcare landscape evolves, CDMOs must prepare for the forthcoming wave of services that will redefine the sector. Key trends include:
- The increasing adoption of artificial intelligence (AI) and machine learning for process optimization
- The rise of personalized medicine
- The growing emphasis on sustainability in manufacturing practices
Slickbit's AI solutions, exemplified by the Trend 483 tool, demonstrate how AI can enhance compliance and operational efficiency by identifying trends in systemic risks and compliance issues derived from FDA 483s.
According to Bikash Chatterjee, "As patient-centered drug design and personalized medicine gain prominence, pharmaceutical companies must navigate more intricate clinical and regulatory landscapes, necessitating greater operational efficiency." This statement underscores the essential role of CDMOs in facilitating these advancements. Furthermore, the CDMO sector is projected to grow at a compound annual growth rate (CAGR) of 9.0% from 2025 to 2032, indicating robust demand for innovative solutions.
Organizations that proactively embrace these trends—including the integration of AI-driven regulatory intelligence and voice agents for operational tasks—will not only enhance their operational capabilities but also position themselves as strategic partners for drug companies seeking to innovate. Staying ahead of these trends is crucial for a CDMO to maintain its competitive edge in a rapidly changing market.
Packaging Solutions: Enhancing Market Readiness through CDMO Partnerships
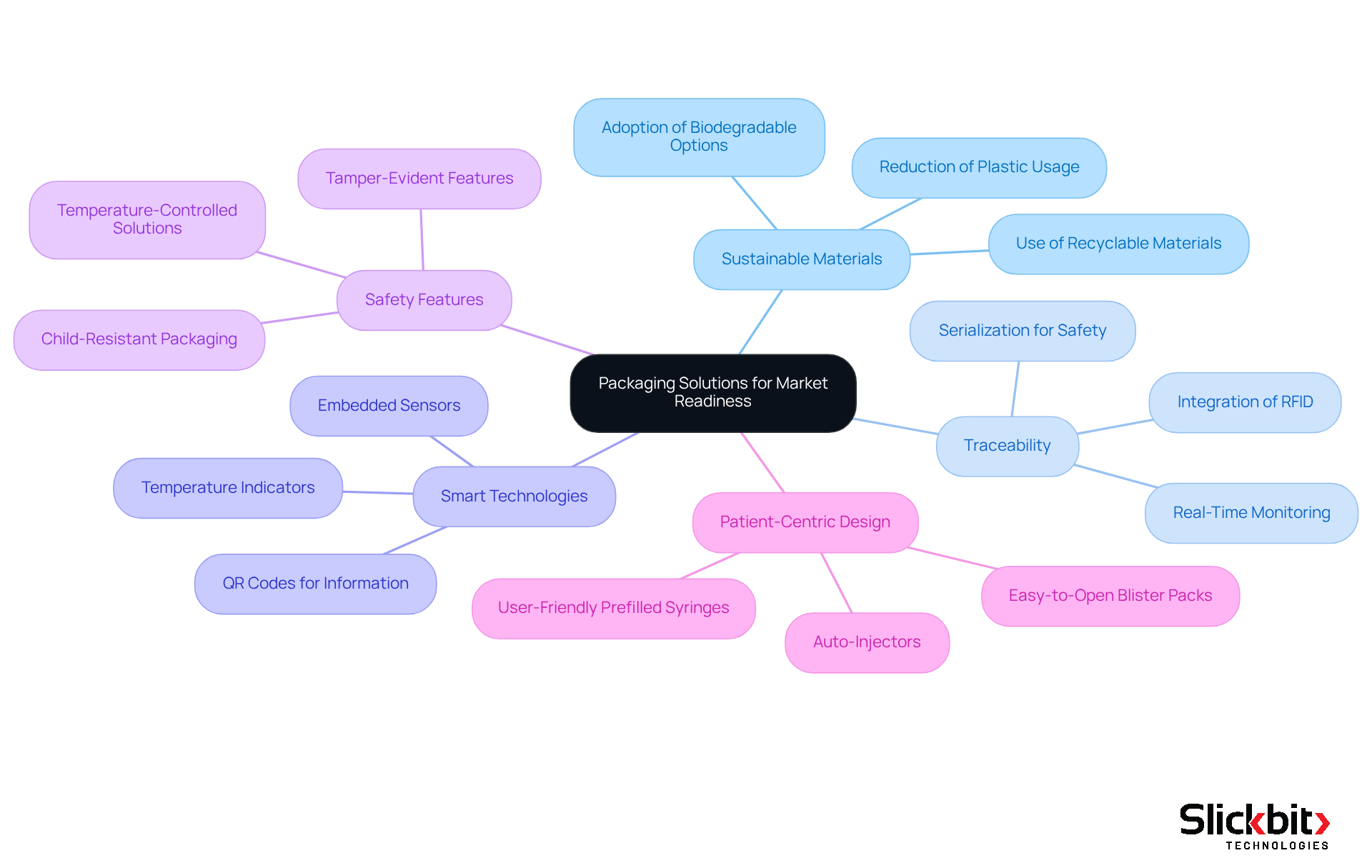
Packaging solutions are essential for enhancing market readiness in the healthcare sector. CDMOs must prioritize innovative packaging strategies that not only protect product integrity but also comply with strict guidelines and align with consumer expectations. This involves:
- The use of sustainable materials
- Ensuring traceability
- Integrating smart packaging technologies, such as sensors and RFID, for real-time monitoring of drug conditions during transport and storage
Furthermore, the adoption of child-resistant and tamper-evident features is becoming standard practice, reflecting the industry's commitment to safety and compliance. Additionally, the growing focus on patient-centric packaging, including user-friendly prefilled syringes and auto-injectors, is reshaping the market by enhancing ease of use and improving the overall patient experience.
By offering comprehensive packaging solutions, a CDMO enables drug companies to streamline product launches and improve market performance, ultimately driving the success of their clients. As Vishakha Agrawal notes, the increasing regulatory demands for sustainable packaging are propelling innovation in the sector, making it imperative for CDMOs to adapt and lead as a CDMO in this evolving landscape.
The pharmaceutical packaging market is projected to grow at a CAGR of 6.16% from 2025 to 2032, underscoring the critical role of innovative packaging strategies in meeting future demands.
Conclusion
The role of Contract Development and Manufacturing Organizations (CDMOs) in pharmaceutical development is increasingly pivotal as the industry navigates complex challenges and opportunities. By implementing strategic approaches such as:
- Rapid Minimum Viable Product (MVP) solutions
- AI-driven regulatory compliance
- Collaborative project management
- Innovative technologies
CDMOs can significantly enhance their operational efficiency and responsiveness to market demands.
Key insights from the article highlight the importance of leveraging advanced analytics and data-driven decision-making to optimize resource allocation and improve project outcomes. Furthermore, the emphasis on quality control practices and effective supply chain management underscores the necessity of maintaining high standards while ensuring timely deliveries. As the landscape evolves, CDMOs must also embrace trends like:
- Personalized medicine
- Sustainable packaging solutions
to remain competitive and relevant.
In conclusion, the future of CDMOs hinges on their ability to adapt to emerging technologies and market trends. By fostering innovation and collaboration, these organizations not only support pharmaceutical companies in overcoming current hurdles but also position themselves as indispensable partners in driving medical advancements. Embracing these strategic approaches will be crucial for CDMOs aiming to thrive in a rapidly changing environment and contribute to the next generation of pharmaceutical development.
Frequently Asked Questions
What is Slickbit and how does it benefit drug development?
Slickbit.ai delivers Minimum Viable Products (MVPs) specifically for Life Sciences companies, enabling Contract Development and Manufacturing Organizations (CDMOs) to accelerate their drug development timelines by rapidly testing innovative concepts while ensuring compliance with industry standards.
How does Slickbit's MVP strategy impact time-to-market?
By focusing on swift MVP creation, Slickbit significantly reduces time-to-market, enhances operational efficiency, and fosters a culture of innovation within CDMO collaborations, allowing organizations to respond effectively to evolving market demands and compliance frameworks.
What is the impact of advanced technologies on MVP development time?
The integration of advanced technologies has been shown to reduce MVP development time by approximately 35%, enabling companies to respond more dynamically to industry challenges.
Can you provide an example of revenue growth from implementing MVP strategies?
Yes, some startups have experienced substantial revenue growth, with annual revenues increasing from $1 million to $20 million within just two years after implementing MVP strategies.
How is AI transforming regulatory compliance for CDMOs?
AI technologies are revolutionizing compliance management by automating compliance monitoring and documentation, which helps mitigate non-compliance risks and enhance operational efficiency within CDMO operations.
What specific AI solutions does Slickbit offer for compliance management?
Slickbit.ai offers AI-powered solutions like Vault Redact, which streamlines the identification and removal of Personally Identifiable Information (PII) and Protected Health Information (PHI) from documents, ensuring secure content management.
What role does Trend 483 play in compliance for CDMOs?
Trend 483 employs AI to identify trends in systemic risks and compliance from FDA 483s, allowing CDMOs to analyze extensive regulatory data effectively, which helps mitigate risks and improves the overall quality of the drug development process.
What is the importance of collaborative project management in CDMO partnerships?
Effective collaborative project management optimizes resource allocation, reduces waste, and enhances productivity by establishing clear communication channels and shared objectives between drug companies and their CDMO partners.
How can project management tools improve collaboration in CDMO partnerships?
Project management tools like ProofHub facilitate real-time data sharing and task tracking, significantly enhancing collaboration and leading to more successful project outcomes and accelerated time-to-market for new drugs.
What best practices can enhance project management success in CDMO operations?
Implementing best practices such as maintaining portfolio lists and facilitating project approval processes can make organizations 2.5 times more successful, highlighting the importance of structured approaches in achieving project goals.




