Overview
This article examines ten software Quality Management System (QMS) solutions that have the potential to significantly enhance research and development (R&D) within the pharmaceutical sector. Notable solutions, such as Slickbit.ai, Veeva Vault QMS, and MasterControl, are specifically designed to elevate compliance, operational efficiency, and process management. These enhancements are essential in addressing the stringent regulatory requirements that characterize the industry, ultimately facilitating a more rapid product development cycle.
Introduction
In the fast-evolving landscape of pharmaceutical research and development, the quest for quality management systems (QMS) has never been more critical. Regulatory requirements are tightening, and the demand for operational efficiency is on the rise. Organizations now face a unique opportunity to leverage innovative software solutions that enhance compliance and streamline processes. However, with numerous options available, companies must discern which QMS solutions best meet their specific needs.
This article explores ten standout software QMS solutions that promise not only to elevate quality management but also to address the pressing challenges currently faced by the pharmaceutical industry.
Slickbit: Custom AI Solutions for Quality Management Systems
Slickbit.ai stands out as a leader in providing customized AI solutions for software QMS specifically tailored for the Life Sciences sector. By emphasizing rapid MVP development, Slickbit enables organizations to quickly implement software QMS solutions that are driven by AI, substantially improving compliance, efficiency, and operational speed. Their proficiency in integrating AI agents into regulatory and clinical workflows allows pharmaceutical companies to enhance process efficiency while adhering to stringent regulatory standards. Furthermore, Slickbit's solutions not only automate routine tasks but also generate actionable insights, which are crucial for R&D managers aiming to refine their improvement practices. This proactive approach to assurance is essential as the pharmaceutical industry navigates evolving regulatory landscapes and seeks to achieve operational excellence.
Veeva Vault QMS: Comprehensive Quality Management for Life Sciences
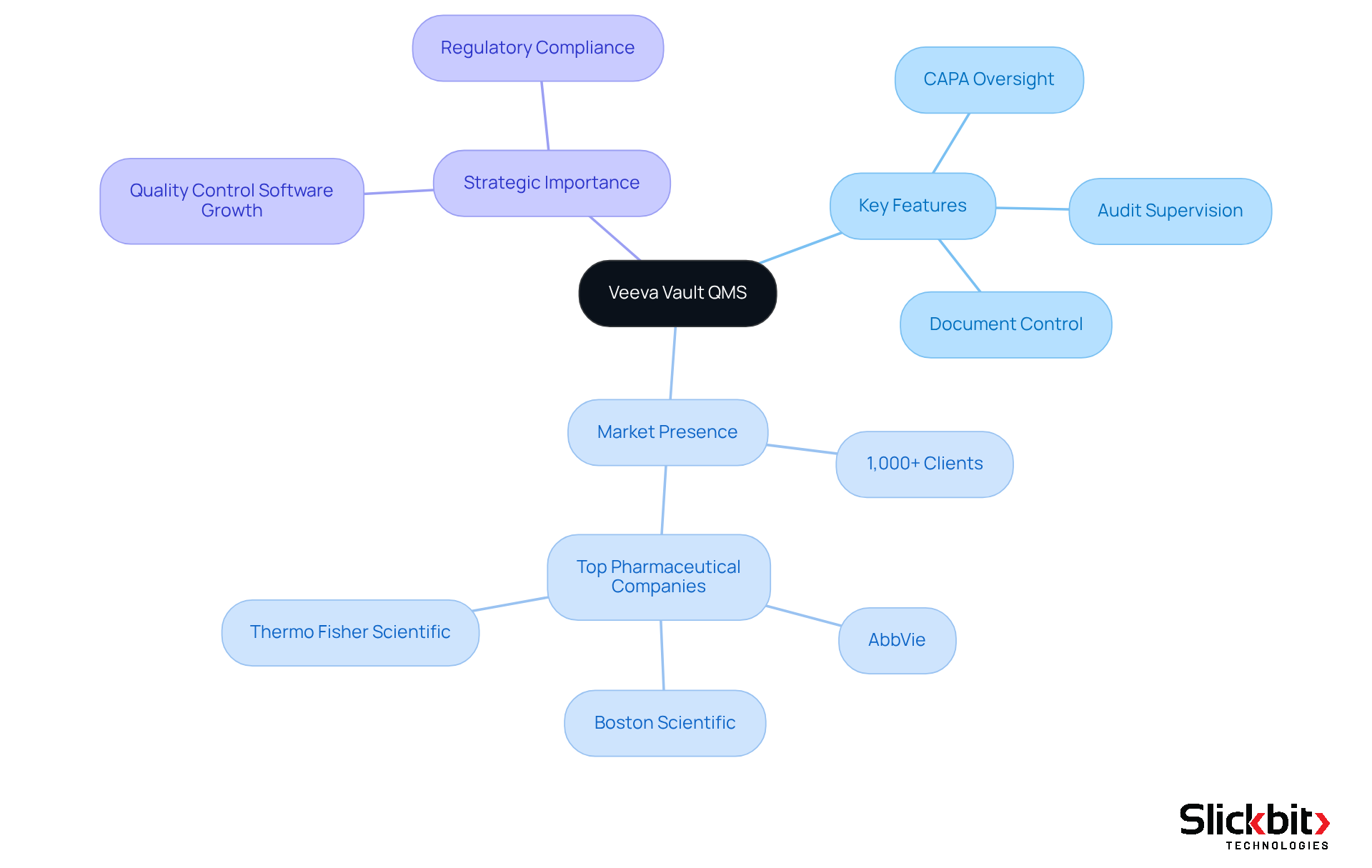
Veeva Vault QMS provides a comprehensive software QMS solution for managing processes that ensure excellence within the Life Sciences sector. This cloud-based platform seamlessly integrates with other Veeva applications, providing a unified approach to oversight. Key features include:
- Document control
- CAPA oversight
- Audit supervision
All designed to enhance compliance and operational efficiency. With over 1,000 clients in the Life Sciences sector, including industry leaders such as AbbVie, Boston Scientific, and Thermo Fisher Scientific, Veeva Vault QMS proves particularly beneficial for organizations aiming to refine their assurance processes while adhering to regulatory requirements. Notably, six of the top twenty global pharmaceutical companies are standardizing on Veeva Vault QMS, underscoring its status as a preferred choice among pharmaceutical firms. As the market for quality control software in the Life Sciences sector is projected to grow significantly, adopting software QMS like Veeva Vault could represent a strategic move for organizations seeking to enhance their quality assurance capabilities.
MasterControl: Advanced Document Control for Compliance
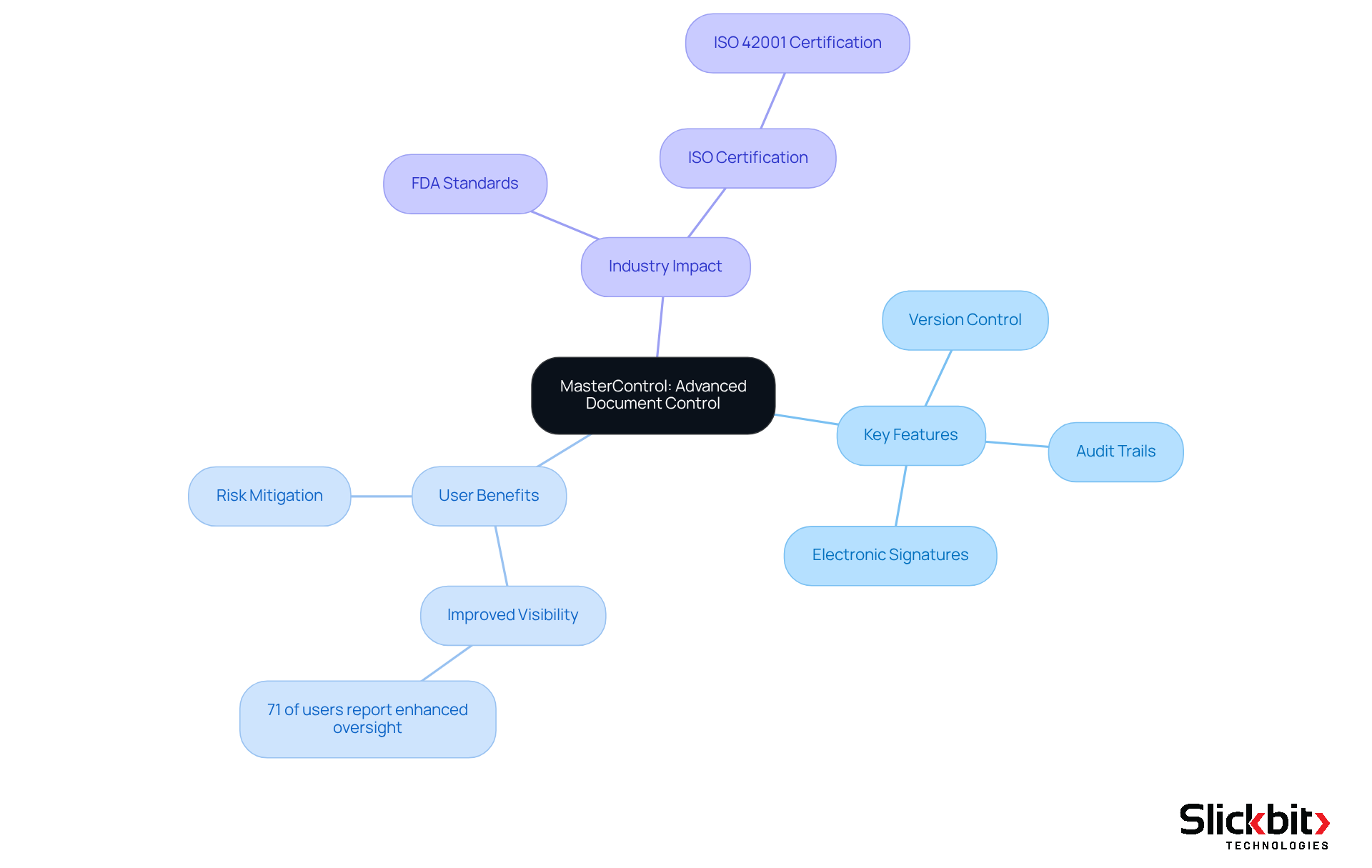
MasterControl delivers advanced document control solutions that are vital for ensuring compliance in regulated industries. Its software QMS automates document handling processes, guaranteeing that all documentation remains current and easily accessible. Key features such as version control, audit trails, and electronic signatures empower organizations to meet FDA and ISO standards effectively.
By streamlining document workflows, MasterControl allows Life Sciences companies to mitigate the risk of non-compliance while enhancing their software QMS and overall quality management. Notably, organizations utilizing MasterControl have reported improved visibility into their regulatory stance, with 71% of users experiencing enhanced oversight, according to a recent study. This capability is crucial as professionals in regulatory fields increasingly emphasize the importance of fostering an ethical culture of adherence, with 76% underscoring its significance in their operations.
Furthermore, MasterControl's recent attainment of ISO 42001 certification highlights its commitment to responsible AI governance, thereby bolstering its credibility within the industry. In addition to MasterControl, Slickbit's AI-powered solutions, such as Vault Redact, automate the identification and removal of PII and PHI from documents, ensuring secure content redaction. Additionally, Trend 483 employs AI to identify trends in systemic risks and adherence from FDA 483s, providing deeper insights that can further enhance operational efficiency.
Advanced document handling systems, such as those offered by MasterControl and Slickbit, are instrumental in strengthening FDA adherence, ultimately fostering a more resilient oversight structure within Life Sciences firms.
Greenlight Guru: Quality Management for Medical Device Companies
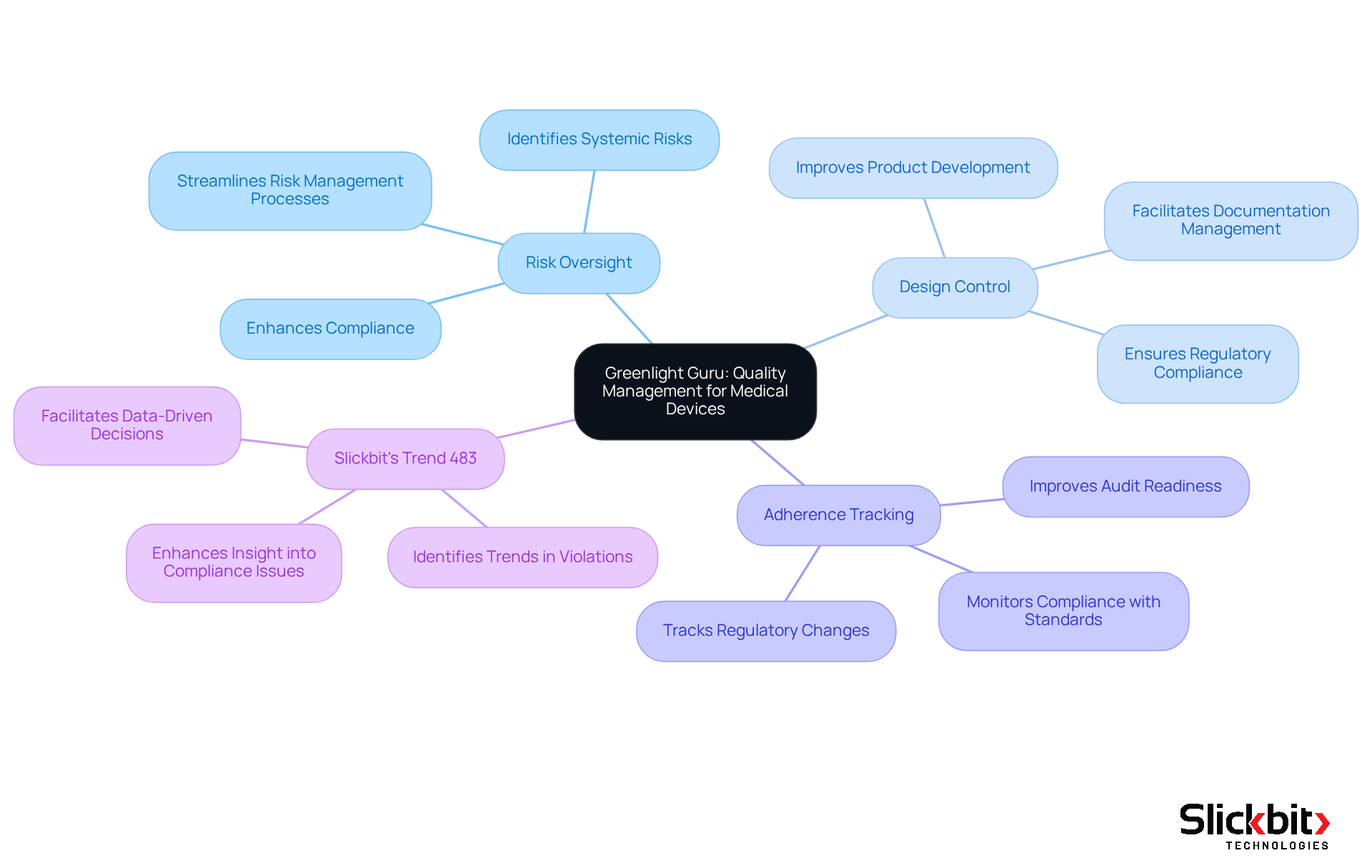
Greenlight Guru stands out as a premier software QMS solution specifically tailored for medical device firms. Its platform encompasses essential features such as:
- Risk oversight
- Design control
- Adherence tracking
These features are meticulously designed to satisfy the rigorous demands of the medical device sector. By offering a centralized system for managing process efficiency, Greenlight Guru empowers organizations to expedite product development while ensuring compliance with regulatory standards.
Furthermore, Slickbit's Trend 483 augments this process by leveraging AI to identify trends in systemic risks and recurring violations from FDA 483s. This innovative tool enables users to search, filter, and access full 483s directly for deeper insights, effectively complementing the capabilities of Greenlight Guru.
With its intuitive interface and robust functionalities, Greenlight Guru serves as an exceptional software QMS choice for firms looking to elevate their standards of practice and navigate the complexities of regulations with confidence.
Qualio: Cloud-Based QMS for Accelerated Compliance
Qualio emerges as a pivotal cloud-based system, significantly accelerating compliance for Life Sciences firms. The software QMS encompasses essential features such as:
- Document control
- Training management
- Corrective and Preventive Action (CAPA) tracking
All meticulously crafted to streamline quality processes. Furthermore, the cloud-based architecture of Qualio enables real-time collaboration among teams, ensuring that all members have immediate access to the most current information. This adaptability proves especially advantageous for organizations striving to scale operations while adhering to stringent regulatory standards.
Notably, Qualio's integration capabilities further enhance operational efficiency, empowering pharmaceutical firms to automate compliance workflows and minimize manual oversight. This is particularly crucial in an industry where developing a new drug can incur costs ranging from USD 1 billion to over USD 2 billion. By leveraging Qualio as a software QMS, companies can not only meet regulatory requirements but also enhance their overall process control. Consequently, this results in expedited product development cycles and heightened patient safety.
TrackWise: Enterprise-Level Quality Management Solutions
TrackWise stands out as a corporate-level solution for excellence, equipped with a robust array of tools designed to oversee procedures across large organizations. Its key features include:
- Corrective and Preventive Action (CAPA) oversight
- Audit coordination
- Risk evaluation
All meticulously crafted to ensure compliance with stringent industry regulations. Furthermore, the scalability of TrackWise makes it an ideal choice for entities of varying sizes, empowering them to enhance their assurance practices in alignment with growth. By integrating effective processes into a unified platform, TrackWise significantly boosts operational efficiency and mitigates the risk of non-compliance. Users have noted its intuitive interface and flexibility, which are essential for maintaining high standards of excellence as companies expand.
Q-Pulse: Flexible QMS Integration for Diverse Industries
Q-Pulse emerges as a highly flexible system for maintaining standards, seamlessly integrating into diverse sectors such as Life Sciences, manufacturing, and healthcare. With extensive functionalities that encompass document handling, training, and incident reporting, it serves as an essential resource for organizations striving to enhance their process standards. This adaptability enables companies to customize their software QMS to comply with specific regulatory requirements, ensuring adherence while concurrently boosting overall efficiency.
Furthermore, as the demand for agile software QMS solutions escalates—particularly in response to evolving regulatory environments—Q-Pulse positions itself as a progressive choice for organizations committed to upholding high standards of excellence and compliance.
ISO 9001: Benchmark for Quality Management Systems
ISO 9001 stands as the premier global standard for excellence systems, applicable to organizations of all sizes and sectors. It provides a robust framework for implementing effective management practices, with a strong emphasis on customer satisfaction and continuous improvement. Organizations that adopt ISO 9001 can significantly enhance their operational efficiency, minimize waste, and elevate customer satisfaction levels. Various software QMS solutions, including those highlighted in this article, are specifically designed to help organizations achieve and maintain ISO 9001 certification, ensuring compliance with international quality standards.
A notable example is Slickbit.ai's Trend 483 tool, which employs artificial intelligence to identify patterns in systemic risks and recurring violations from FDA 483s. This innovative approach offers deeper insights that can bolster adherence efforts. As industry leaders assert, continuous improvement within the software QMS is crucial for sustaining competitive advantage and adapting to evolving market demands. By leveraging AI-driven insights from tools like Trend 483, organizations can further optimize their operations and refine their regulatory strategies.
ETQ Reliance: Cloud-Based QMS for Risk Management
ETQ Reliance stands out as a cloud-based software QMS dedicated to ensuring standards, emphasizing risk oversight as a core functionality. This platform equips organizations with robust tools for managing significant events and corrective and preventive actions (CAPA), all designed to proactively address potential risks through software QMS. By integrating risk oversight into excellence processes, ETQ Reliance markedly enhances overall governance practices and compliance with stringent regulatory requirements through the use of software QMS. This focus is especially critical for Life Sciences firms, which operate within complex regulatory frameworks and must maintain high standards of adherence.
As the pharmaceutical industry increasingly adopts ETQ Reliance, its software QMS capabilities in risk oversight become essential for navigating the evolving landscape of assurance standards. Furthermore, Slickbit.ai's Vault Redact significantly improves document redaction processes by automating the identification and removal of PII and PHI, thus ensuring compliance with rigorous regulations. Notably, the pharmaceutical sector accounted for over 57% of the revenue share in the life sciences assurance software market in 2024, underscoring the importance of such systems.
In addition, the recent launch of ETQ Reliance® Predictive Quality Analytics illustrates the platform's innovative approach to enhancing oversight through artificial intelligence. Insights gleaned from AI transformations in other sectors, such as the restaurant industry, can provide valuable lessons for pharmaceutical companies aiming to leverage AI for improved adherence and operational efficiency.
Oracle Quality Management Cloud: Integrated Solutions for Operational Efficiency
Slickbit.ai offers a suite of AI-driven solutions designed to significantly enhance operational efficiency and compliance throughout the management process in the pharmaceutical sector. One of the standout features, Vault Redact, automates the identification and removal of personally identifiable information (PII) and protected health information (PHI) from documents, ensuring secure content redaction. Furthermore, Trend 483 leverages AI to pinpoint trends in systemic risks and compliance issues stemming from FDA 483s, enabling teams to search, filter, and gain deeper insights into regulatory challenges.
By harnessing advanced analytics and real-time information, organizations can proactively identify and address issues related to standards, thereby mitigating risks associated with non-compliance. This integrated approach not only streamlines excellence processes but also fosters continuous improvement initiatives, making software qms an indispensable asset for Life Sciences firms.
For instance, organizations that have adopted these tools report significant enhancements in productivity and efficiency, ultimately facilitating quicker patient access to therapies and bolstering market positioning. As a Slickbit executive noted, 'The integration of our AI solutions allows companies to not only meet regulatory demands but also to enhance their operational capabilities, ensuring that quality is built into every step of the process.
Conclusion
The exploration of software Quality Management Systems (QMS) tailored for the pharmaceutical sector reveals a transformative landscape where efficiency and compliance are paramount. By adopting these innovative solutions, organizations can significantly enhance their operational capabilities, ensuring they meet the rigorous standards of the industry while accelerating product development cycles.
Key players such as Slickbit, Veeva Vault, and MasterControl have been highlighted for their unique contributions to quality management. Each solution offers distinct features—from AI-driven insights and document control to risk management and compliance tracking—that empower pharmaceutical firms to streamline processes and foster a culture of continuous improvement. The integration of these systems not only mitigates risks associated with non-compliance but also enhances overall productivity, paving the way for quicker patient access to essential therapies.
In a rapidly evolving regulatory environment, the importance of robust software QMS solutions cannot be overstated. Organizations are urged to evaluate their quality management strategies and consider the adoption of these advanced systems. By doing so, they can ensure adherence to industry standards while positioning themselves for future success in the competitive pharmaceutical landscape. Embracing these technologies will ultimately lead to improved patient outcomes and a stronger commitment to quality in every aspect of pharmaceutical research and development.
Frequently Asked Questions
What is Slickbit and what does it offer for the Life Sciences sector?
Slickbit is a provider of customized AI solutions specifically designed for software Quality Management Systems (QMS) in the Life Sciences sector. It focuses on rapid MVP development to help organizations quickly implement AI-driven QMS solutions, improving compliance, efficiency, and operational speed.
How does Slickbit enhance process efficiency for pharmaceutical companies?
Slickbit integrates AI agents into regulatory and clinical workflows, which helps pharmaceutical companies enhance process efficiency while adhering to strict regulatory standards. Their solutions automate routine tasks and generate actionable insights for R&D managers.
What is Veeva Vault QMS and what features does it offer?
Veeva Vault QMS is a comprehensive cloud-based software solution for managing quality processes in the Life Sciences sector. Key features include document control, CAPA oversight, and audit supervision, all aimed at enhancing compliance and operational efficiency.
Who are some of the clients using Veeva Vault QMS?
Veeva Vault QMS is utilized by over 1,000 clients in the Life Sciences sector, including industry leaders such as AbbVie, Boston Scientific, and Thermo Fisher Scientific. Six of the top twenty global pharmaceutical companies are also standardizing on Veeva Vault QMS.
What benefits does MasterControl provide for compliance in regulated industries?
MasterControl offers advanced document control solutions that automate document handling processes, ensuring that documentation is current and accessible. Key features include version control, audit trails, and electronic signatures, which help organizations meet FDA and ISO standards effectively.
What impact does MasterControl have on regulatory compliance visibility?
Organizations using MasterControl have reported improved visibility into their regulatory compliance, with 71% of users experiencing enhanced oversight, which is crucial for fostering an ethical culture of adherence in regulated industries.
What recent certification has MasterControl achieved?
MasterControl has recently attained ISO 42001 certification, which demonstrates its commitment to responsible AI governance and enhances its credibility within the industry.
How do Slickbit's AI-powered solutions contribute to document security?
Slickbit's AI-powered solutions, such as Vault Redact, automate the identification and removal of Personally Identifiable Information (PII) and Protected Health Information (PHI) from documents, ensuring secure content redaction.
What is the role of Trend 483 in operational efficiency?
Trend 483 employs AI to identify trends in systemic risks and adherence from FDA 483s, providing deeper insights that can enhance operational efficiency within Life Sciences firms.
Why are advanced document handling systems important for Life Sciences companies?
Advanced document handling systems, like those offered by MasterControl and Slickbit, are essential for strengthening FDA adherence, thereby fostering a more resilient oversight structure within Life Sciences organizations.




