Overview
This article examines the diverse regulatory affairs job opportunities available in Bangalore for R&D managers, underscoring the critical importance of compliance roles within the life sciences sector. The rising demand for skilled professionals in regulatory affairs is compelling, as leading companies such as AbbVie, Philips, and Teva Pharmaceuticals actively seek candidates who possess the necessary expertise in compliance. These professionals are essential for navigating complex governance frameworks and ensuring adherence to the ever-evolving regulatory standards. Consequently, the landscape for regulatory affairs positions is not just expanding; it is becoming increasingly vital for the success of organizations in this sector.
Introduction
The regulatory affairs landscape in Bangalore is evolving rapidly, driven by the increasing complexity of compliance requirements and the integration of advanced technologies. As organizations strive to meet stringent global standards, the demand for skilled R&D managers in regulatory affairs is on the rise, presenting a wealth of opportunities for professionals in this field. However, with the landscape shifting due to AI innovations and regulatory changes, candidates must effectively position themselves to secure these coveted roles. This article delves into the top regulatory affairs job openings in Bangalore, offering insights into the skills, responsibilities, and expectations that define this dynamic sector.
Slickbit: AI Solutions for Regulatory Affairs Compliance
Slickbit.ai is at the forefront of developing AI solutions that significantly enhance the capabilities of compliance teams within life sciences organizations. By leveraging advanced AI analytics and insights, Slickbit streamlines adherence procedures, enabling organizations to adeptly navigate the complexities of governance frameworks. The rapid development of Minimum Viable Products (MVPs) empowers R&D managers to swiftly validate AI use cases, thereby boosting productivity and ensuring compliance in submissions and reporting.
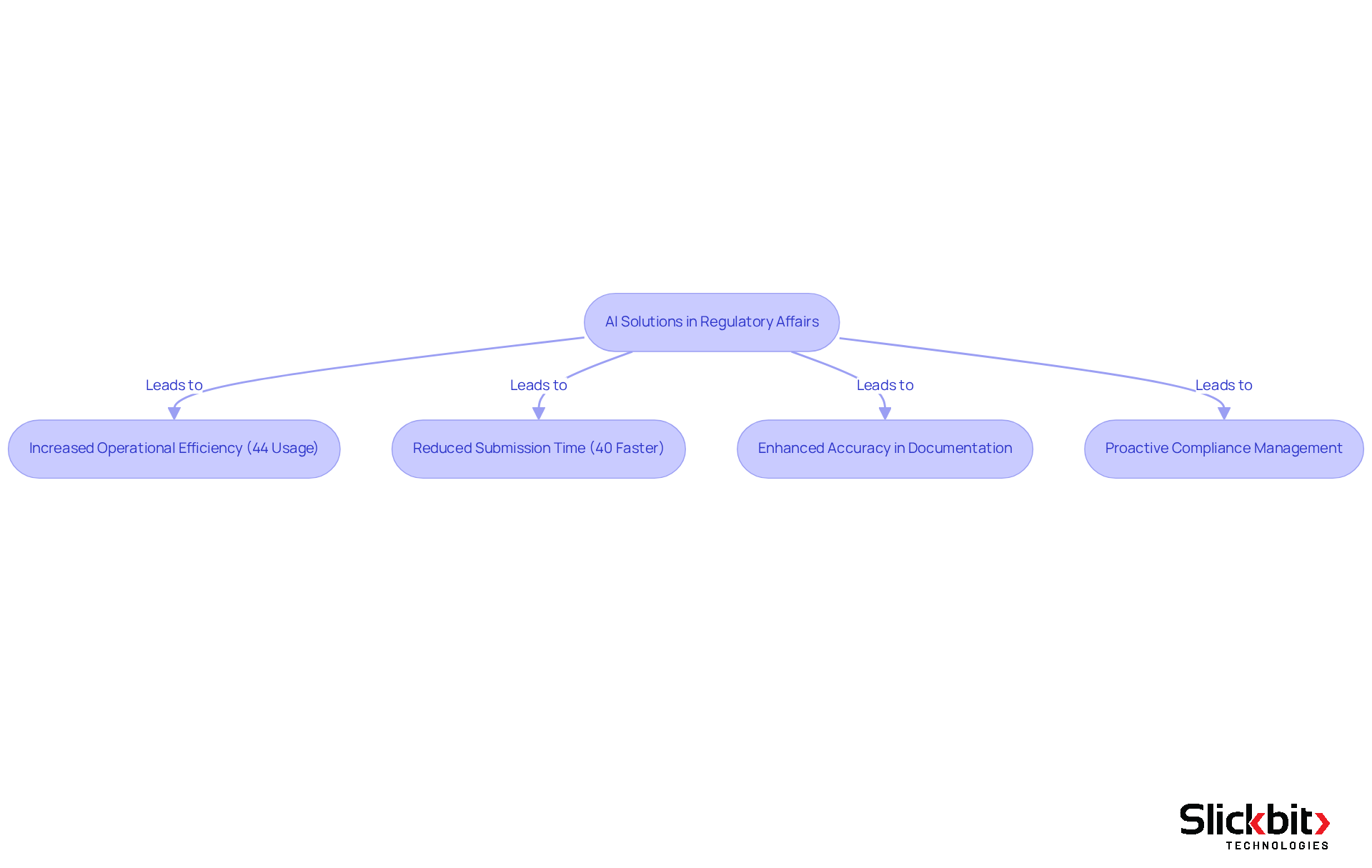
Experts in the field assert that AI is revolutionizing regulatory processes, with 44% of regulatory officers already utilizing AI tools to improve operational efficiency. The integration of AI not only accelerates the submission process—potentially reducing it by 40%—but also enhances submission accuracy, significantly minimizing documentation errors. As AI continues to evolve, its role in oversight is expected to expand, fostering a more proactive approach to compliance management.
Successful applications of AI analytics in compliance submissions have revealed considerable time savings and improved accuracy, allowing teams to focus on strategic risk management rather than manual data gathering. This transition is crucial as the pharmaceutical industry faces increasing oversight demands and the need for adaptable compliance strategies. With AI-driven insights, compliance professionals can better anticipate inquiries from agencies like the FDA, facilitating smoother review processes and expediting approvals.
AbbVie: Senior Manager, Regulatory Affairs in Bangalore
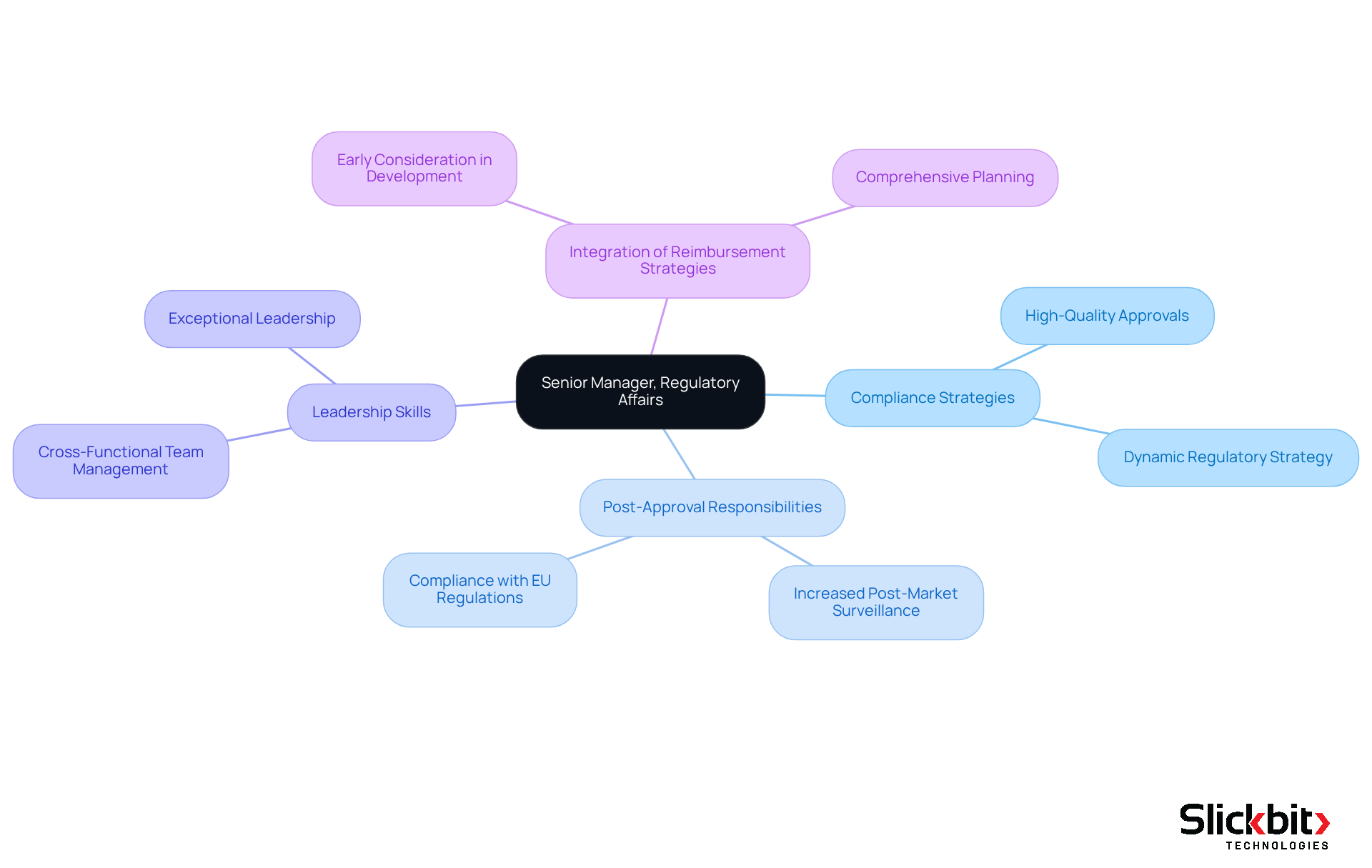
AbbVie is actively seeking a Senior Manager for Compliance Affairs in Bangalore, a pivotal role focused on developing strategies that facilitate high-quality approvals for new pharmaceutical products while overseeing post-approval responsibilities. Ideal candidates will possess substantial experience in compliance affairs, particularly within the pharmaceutical sector, and must exhibit exceptional leadership skills to effectively navigate cross-functional teams through intricate compliance landscapes. This role necessitates not only a deep understanding of legal frameworks but also the ability to formulate strategies that align with evolving market dynamics and compliance requirements.
A meticulously crafted oversight strategy is vital for planning, budgeting, and timing, especially in light of increased post-market surveillance demands in the EU. As industry leader Mike Drues asserts, "It’s never too soon, in my opinion, to start thinking about compliance strategy," underscoring the proactive mindset essential for this position.
Furthermore, candidates should appreciate the importance of integrating reimbursement strategies with compliance strategies early in the product development process to ensure comprehensive planning.
Philips: Regulatory Affairs Excellence Specialist Role
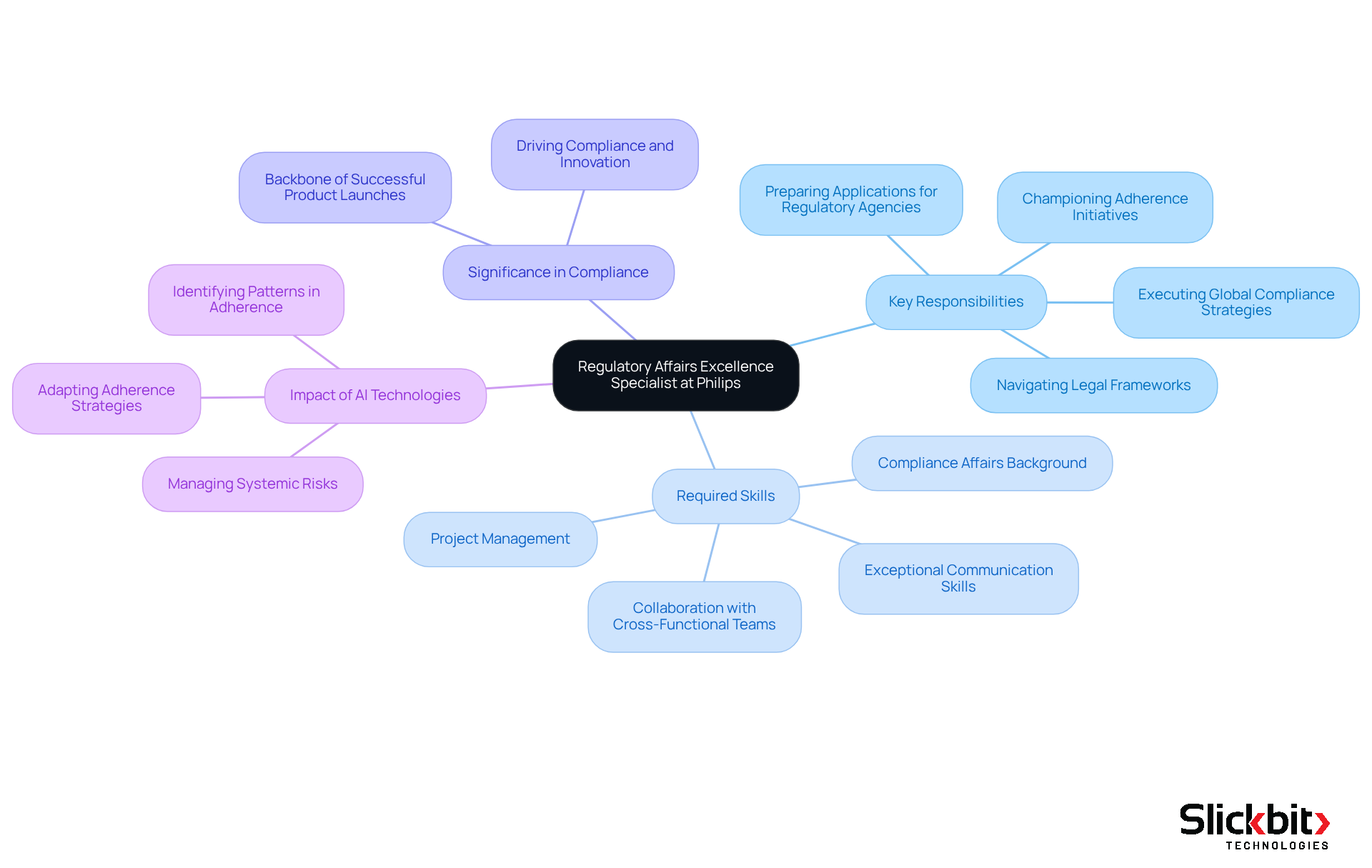
Philips is actively seeking regulatory affairs jobs in Bangalore, specifically for a Regulatory Affairs Excellence Specialist, a vital position for executing global compliance strategies and ensuring adherence to medical device standards. This role demands a strong background in compliance affairs, complemented by exceptional communication skills and the ability to collaborate effectively with cross-functional teams. It not only champions adherence initiatives but also plays a crucial role in navigating the complexities of legal frameworks, ensuring that Philips remains at the forefront of conformity within the healthcare sector.
As governance environments evolve, particularly with the integration of AI technologies that identify patterns in adherence and systemic risks, the ability to adapt and implement effective adherence strategies will be essential for the success of medical devices in the marketplace. Furthermore, as one Regulatory Affairs Specialist noted, 'The significance of adherence strategies cannot be overstated; they are the backbone of successful product launches in the healthcare sector.' This underscores the importance of this role in driving compliance and fostering innovation.
Teva Pharmaceuticals: Regulatory Affairs Associate III Position
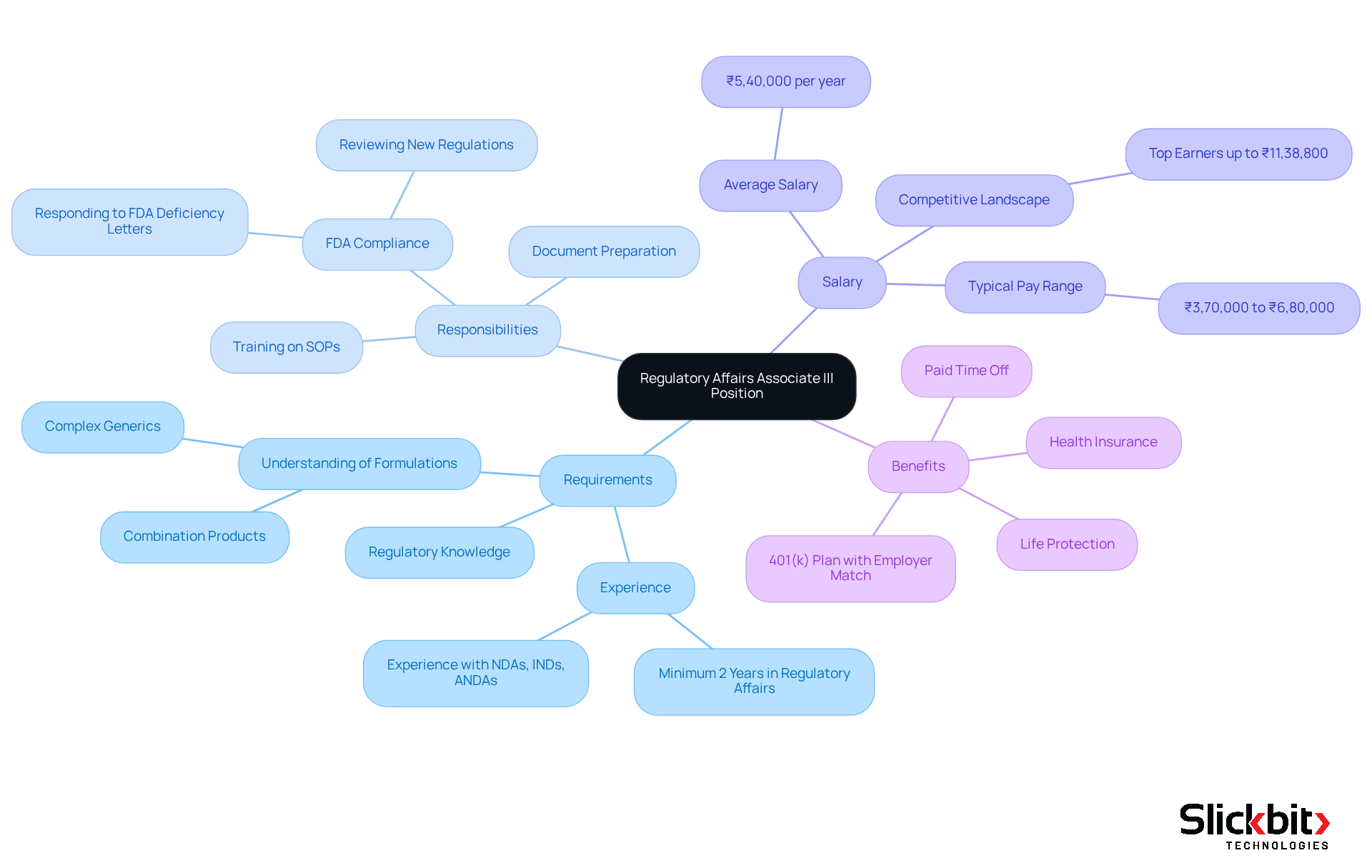
Teva Pharmaceuticals is actively seeking regulatory affairs jobs in Bangalore, particularly for the role of Regulatory Affairs Associate III, which is crucial for the preparation and review of submission documents to ensure compliance with FDA guidelines. Candidates must demonstrate a strong grasp of regulatory requirements and possess substantial experience within the pharmaceutical sector, particularly in managing submissions for new drug applications (NDAs). A thorough understanding of various formulations, including complex generics and combination products, is essential for this position.
As of 2025, the average salary for this role in Bangalore is approximately ₹5,40,000 per year, with a typical pay range between ₹3,70,000 and ₹6,80,000, reflecting the competitive landscape of the industry. Successful candidates will excel in navigating the complexities of FDA regulations, including recent updates pertinent to new drug applications, and will play a crucial role in maintaining approved applications. Furthermore, this role entails training on internal Standard Operating Procedures (SOPs), which is vital for ensuring compliance.
Insights from industry specialists underscore the importance of meticulous documentation and adherence to standards, which are critical for the effective management of NDAs in oversight matters. Additionally, leveraging AI tools such as Trend 483 can provide valuable insights into systemic risks and compliance trends, thereby enhancing the efficiency of oversight processes. In addition to a competitive salary, Teva offers a comprehensive benefits package, including life and disability protection, paid time off, and health insurance commencing on the first day of employment.
Baxter: Regulatory Affairs Jobs in Bangalore
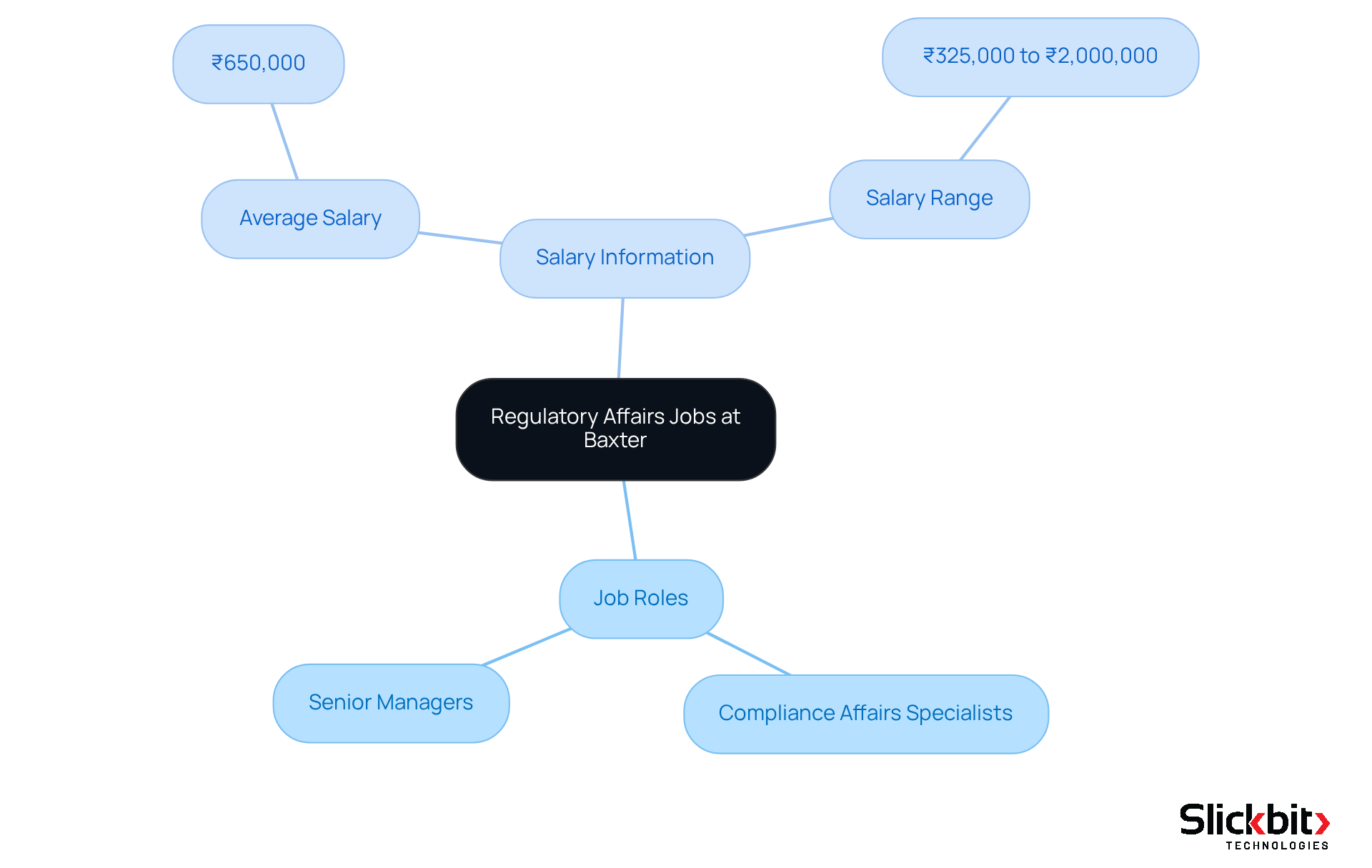
Baxter is actively seeking applicants for a variety of regulatory affairs jobs in Bangalore, including roles for compliance affairs specialists and senior managers. These positions play a crucial role in ensuring adherence to increasingly stringent global regulations for medical devices, set to take effect in 2025.
Candidates are expected to have a robust background in submissions and a comprehensive understanding of the healthcare sector's compliance landscape. Typically, compliance specialists in Bangalore earn around ₹650,000 annually, with salaries ranging from ₹325,000 to ₹2,000,000, reflecting variability based on experience and position.
As one compliance specialist noted, adhering to international standards is not merely a necessity; it is a commitment to excellence and safety in healthcare.
Furthermore, Baxter encourages job seekers to explore their current openings and consider joining their Talent Community to stay connected and learn more about the company.
BD: Associate Staff Regulatory Affairs Specialist Opportunities
BD is currently offering regulatory affairs jobs in Bangalore for Associate Staff Regulatory Affairs Specialists. These roles are essential for overseeing compliance activities for existing products, ensuring adherence to the rapidly evolving laws in the life sciences sector. Candidates must possess a strong background in life sciences along with relevant experience in compliance matters. Furthermore, robust analytical and communication skills are vital for effectively navigating the complexities of compliance environments.
The average salary for this position in Bangalore is approximately ₹540,000, with a range between ₹373,000 and ₹680,000, reflecting the increasing demand for skilled professionals in this field.
As the oversight environment continues to evolve, particularly with the integration of AI technologies that enhance adherence monitoring and operational productivity, industry leaders emphasize the importance of staying informed about adherence requirements. This vigilance not only mitigates risks but also boosts operational efficiency.

Biocon Biologics: Regulatory Affairs Positions in Bangalore
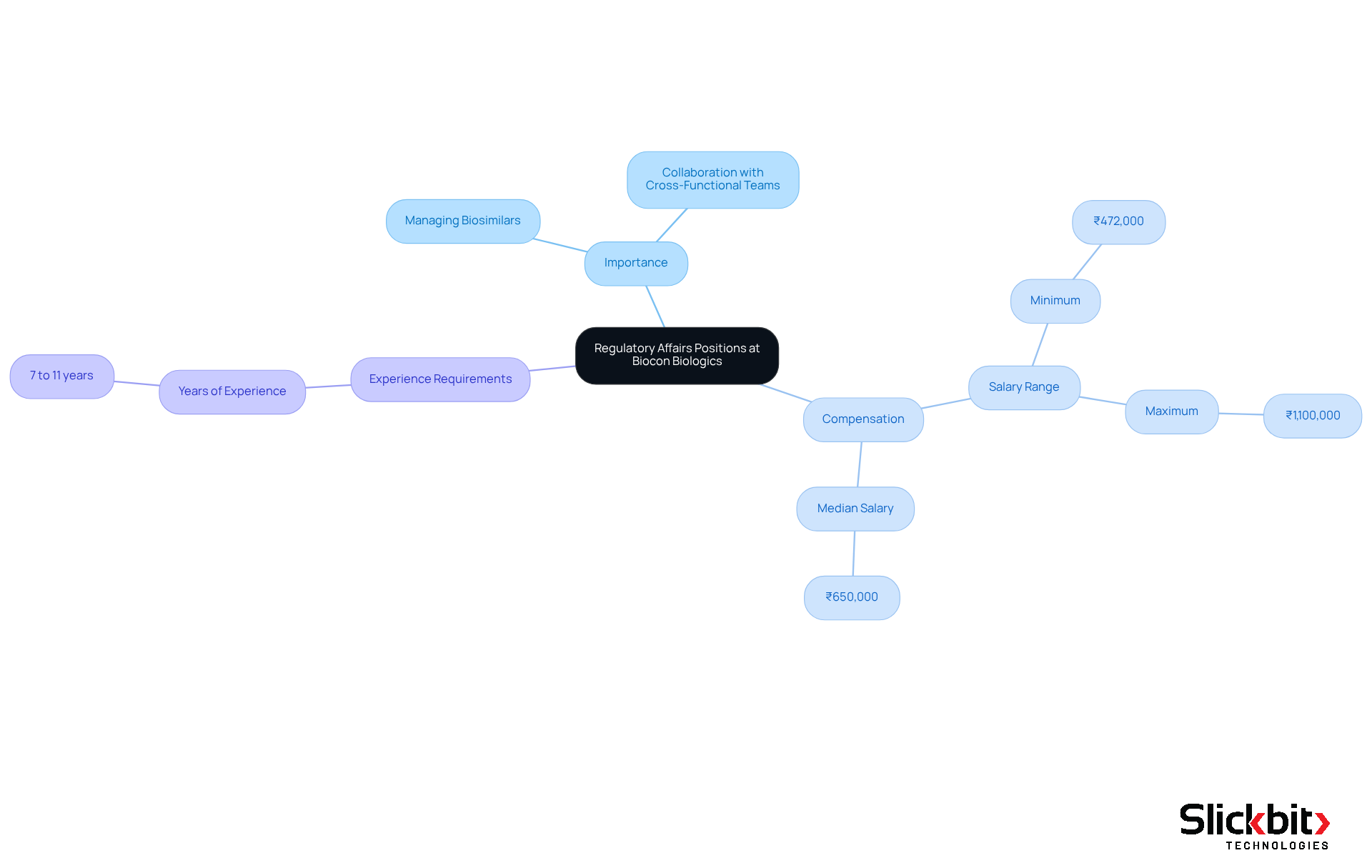
Biocon Biologics is actively pursuing candidates for regulatory affairs jobs in Bangalore, highlighting the critical importance of managing submissions for biosimilars. These roles require applicants to possess a robust background in oversight matters, particularly within the biologics sector, and demonstrate proficiency in navigating challenges associated with international standards. Successful candidates will collaborate with cross-functional teams to meet compliance objectives, ensuring alignment with the evolving requirements anticipated in 2025.
The compensation for compliance specialists in the biologics field in Bangalore ranges from ₹472,000 to ₹1,100,000 annually, with a median total compensation of ₹650,000. This range reflects the significant demand for expertise in this area. Moreover, certain positions may require 7 to 11 years of experience, highlighting the stringent qualifications needed for compliance roles at Biocon Biologics.
AstraZeneca: Regulatory Affairs Job Opportunities
AstraZeneca is actively seeking qualified candidates for regulatory affairs jobs in Bangalore, focusing on the development of compliance strategies and the preparation of submissions to ensure adherence to global standards. These positions are crucial as the pharmaceutical sector faces increasing scrutiny due to rapid country-specific policy changes and a lack of global harmonization expected in 2025.
Successful candidates will possess a robust understanding of the compliance landscape and relevant experience in oversight, enabling them to manage complex submission processes effectively. This includes ensuring that clinical trial data and compliance submissions are meticulously prepared to minimize additional review cycles with regulatory authorities.
With the average compensation for compliance specialists in regulatory affairs jobs in Bangalore at AstraZeneca projected to be competitive, these regulatory affairs jobs in Bangalore present an excellent opportunity for professionals aiming to advance their careers in a dynamic environment.
As companies adapt to more stringent compliance requirements and the need for efficient submissions in international markets, the demand for skilled professionals in compliance continues to grow. Candidates are encouraged to engage with compliance advisors and participate in FDA advisory sessions to enhance their knowledge and readiness for these positions.
Parexel: Regulatory Affairs Roles in Clinical Research
Parexel is actively seeking qualified candidates for various compliance roles within the realm of clinical research. These positions are vital for preparing and reviewing submissions for clinical trials, ensuring strict adherence to compliance standards. Candidates who possess a robust background in compliance, complemented by practical experience in clinical research, will find themselves well-suited for these roles. Furthermore, strong communication skills are essential for effective collaboration with cross-functional teams, as these specialists play a critical role in navigating the complexities of compliance requirements.
In 2025, the average salary for regulatory affairs jobs in Bangalore is projected to be competitive, reflecting the escalating demand for expertise in this domain. Regulatory adherence stands as a cornerstone of clinical trials, underscoring its crucial role in safeguarding patient safety and ensuring the integrity of research outcomes. Insights from industry professionals highlight that upholding high compliance standards transcends mere obligation; it represents a commitment to advancing healthcare and enhancing patient outcomes.
Syngene International: Regulatory Affairs Job Openings
Syngene International is actively seeking applicants for compliance roles in Bangalore, underscoring the vital responsibility of overseeing submissions and ensuring adherence to industry standards in drug development. Candidates with a robust background in regulatory affairs jobs in Bangalore will be favored, showcasing a comprehensive understanding of the dynamic regulatory landscape within the life sciences sector.
Insights from industry leaders emphasize the necessity of strict compliance, as organizations navigate intricate submission processes. Furthermore, leveraging AI tools such as Trend 483 can offer invaluable insights into FDA compliance, enabling professionals to identify trends in systemic risks and recurring violations, thus enhancing operational efficiency. Trend 483 empowers users to search, filter, and access complete 483s for deeper insights, which is crucial for compliance professionals.
In 2025, the anticipated average compensation for compliance professionals at Syngene International is expected to be competitive, reflecting the growing demand for expertise in this field. As governance structures continue to evolve, specialists in this domain will play a pivotal role in facilitating effective drug development and ensuring compliance. Recent challenges, including the FDA's staffing reductions, may affect regulatory submissions, thereby amplifying the importance of regulatory affairs jobs in Bangalore for ensuring timely and compliant drug development.
Conclusion
The landscape of regulatory affairs jobs in Bangalore is undergoing a significant transformation, propelled by technological advancements and the escalating compliance demands within the life sciences sector. The integration of AI solutions is redefining the operations of compliance teams, empowering them to navigate complex regulations with enhanced efficiency and precision. This evolution not only boosts productivity but also ensures that organizations can adeptly meet stringent oversight requirements.
Throughout the article, notable companies such as AbbVie, Philips, and Teva Pharmaceuticals have underscored the vital roles available in regulatory affairs. Each position underscores the necessity for a robust understanding of compliance frameworks, strategic planning capabilities, and the aptitude for cross-team collaboration. As the industry encounters increased scrutiny and the imperative for flexible compliance strategies, the demand for skilled professionals is poised for substantial growth.
In conclusion, pursuing a career in regulatory affairs in Bangalore offers a promising avenue for professionals eager to make a meaningful impact in the life sciences field. With the convergence of AI technologies and the evolving regulatory landscape, candidates are strongly encouraged to remain informed about industry trends and to continuously refine their skills. Engaging with companies actively seeking talent in these roles can significantly enhance prospects for a successful career in this dynamic and essential sector.
Frequently Asked Questions
What is Slickbit's role in regulatory affairs compliance?
Slickbit.ai develops AI solutions that enhance compliance teams' capabilities within life sciences organizations, streamlining adherence procedures and helping organizations navigate governance frameworks.
How does Slickbit utilize AI in compliance processes?
Slickbit leverages advanced AI analytics to boost productivity, validate AI use cases, and improve compliance in submissions and reporting, potentially reducing submission processes by 40% and enhancing accuracy.
What benefits have been observed from using AI in regulatory processes?
Successful applications of AI analytics have resulted in considerable time savings and improved accuracy in compliance submissions, allowing teams to focus on strategic risk management rather than manual data gathering.
What is the significance of the Senior Manager position at AbbVie?
The Senior Manager for Compliance Affairs at AbbVie is responsible for developing strategies for high-quality approvals of new pharmaceutical products and overseeing post-approval responsibilities, requiring substantial experience in compliance affairs.
What skills are essential for the Senior Manager role at AbbVie?
Candidates must have exceptional leadership skills, a deep understanding of legal frameworks, and the ability to formulate strategies that align with evolving market dynamics and compliance requirements.
Why is a proactive compliance strategy important in the pharmaceutical industry?
A proactive compliance strategy is crucial due to increased post-market surveillance demands, allowing for effective planning, budgeting, and timing in compliance management.
What is the focus of the Regulatory Affairs Excellence Specialist role at Philips?
The Regulatory Affairs Excellence Specialist at Philips is responsible for executing global compliance strategies and ensuring adherence to medical device standards, requiring a strong background in compliance affairs.
How does the integration of AI technologies impact regulatory affairs at Philips?
AI technologies help identify patterns in adherence and systemic risks, making it essential for regulatory affairs specialists to adapt and implement effective adherence strategies for successful medical device launches.
What is the importance of adherence strategies in regulatory affairs?
Adherence strategies are crucial as they form the backbone of successful product launches in the healthcare sector, ensuring compliance and fostering innovation.




