Overview
This article addresses the critical strategies necessary to circumvent prevalent pitfalls in Individual Case Safety Reports (ICSR) reporting. It underscores the paramount importance of accuracy, compliance, and a commitment to continuous improvement in this domain. Key strategies outlined include:
- The adoption of AI-driven tools for data validation
- The establishment of clear reporting timelines
- Investment in comprehensive training programs
- The necessity of staying abreast of regulatory requirements
Each of these elements is essential for enhancing the quality and timeliness of ICSR submissions, ultimately leading to improved outcomes and compliance within the industry.
Introduction
In the intricate world of pharmacovigilance, the accuracy of Individual Case Safety Reports (ICSR) is paramount. However, organizations frequently encounter significant reporting pitfalls that jeopardize patient safety and regulatory compliance. This article delves into ten essential strategies designed to help organizations navigate these challenges, enhance their reporting practices, and ultimately foster a culture of accountability. As the landscape of ICSR reporting evolves, the pressing question arises: how can companies ensure they are not only compliant but also ahead of the curve in maintaining data integrity and safety?
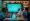
Slickbit: AI Solutions for Enhancing ICSR Reporting Accuracy
Slickbit.ai stands at the forefront of developing AI-driven solutions that significantly enhance the accuracy of ICSR. By leveraging advanced analytics and machine learning, Slickbit empowers Life Sciences organizations to optimize their documentation processes, thereby ensuring compliance with regulatory standards. Furthermore, the swift advancement of enterprise-level AI Minimum Viable Products (MVPs) allows teams to promptly implement customized AI solutions that address specific challenges, such as information validation and error reduction.
For instance, the AI-driven regulatory intelligence assistant Lumino provides precise, traceable responses from FDA guidance documents, directly improving the accuracy of ICSR submissions. This proactive approach results in more reliable safety data submissions, enhancing overall compliance and efficiency in pharmacovigilance practices. Recent advancements in AI technologies, including automation tools like Vault Redact, facilitate the identification and removal of sensitive information, further bolstering compliance efforts.
However, it is essential to remain cognizant of the potential risks associated with AI, particularly the 'black-box' nature of AI models, which can complicate transparency and accountability in pharmacovigilance.
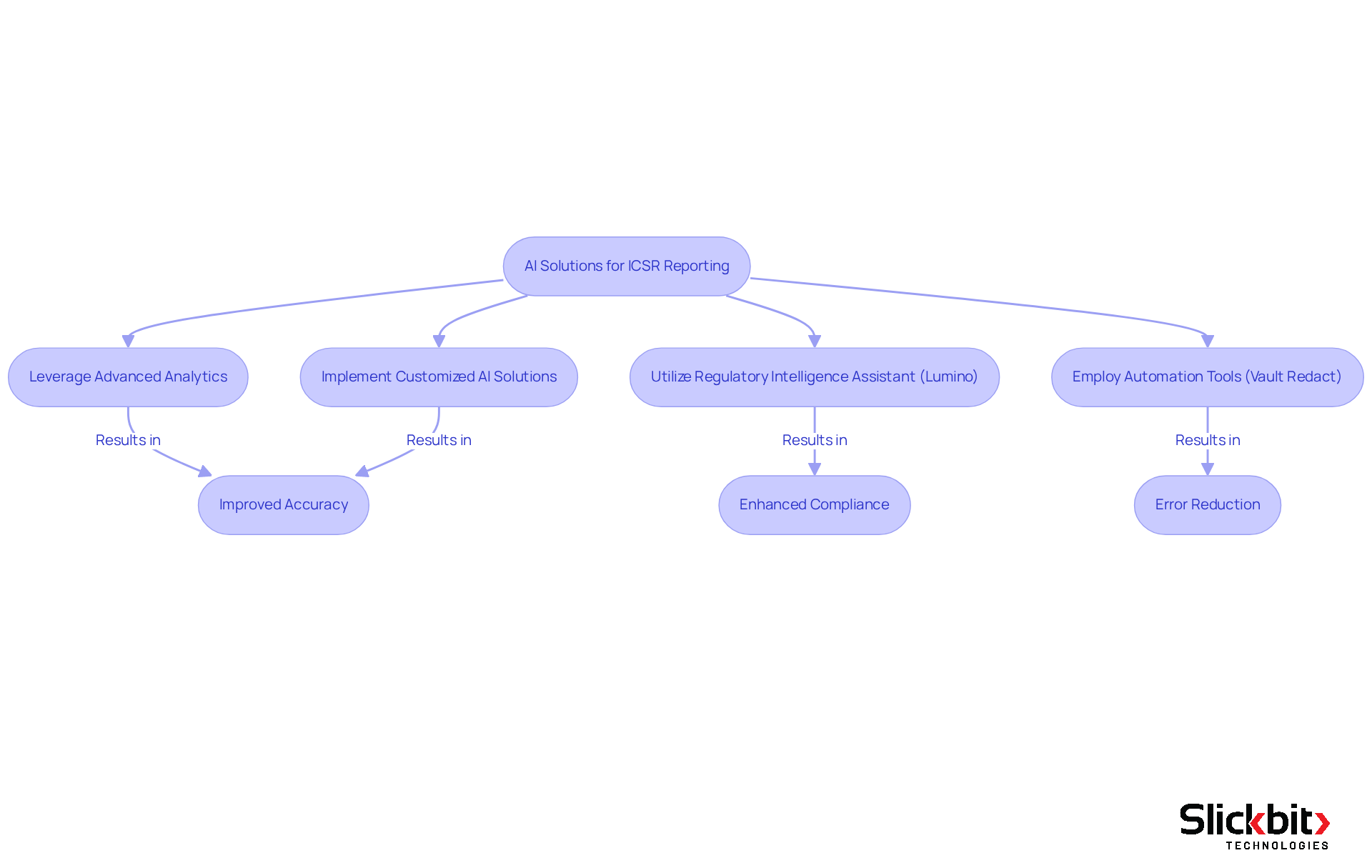
Misclassification of Adverse Events: Understanding the Risks
Misclassification of adverse events presents a significant threat to patient safety and regulatory compliance. Common pitfalls include:
- Conflating adverse reactions with unrelated medical conditions
- Inaccurately assessing the severity of incidents
Such misclassifications can result in flawed risk evaluations and potential regulatory penalties, thereby undermining the integrity of safety documentation systems. For instance, 9.9% of adverse events were not reported to the FDA within the mandated 15 days, underscoring the critical need for accurate classification.
To address these challenges, entities must develop comprehensive training initiatives focused on the nuances of negative event documentation. These programs should incorporate:
- Role-playing scenarios
- Workshops
- Case studies that reinforce just culture principles and the importance of precise documentation
Continuous education is essential for staff to differentiate between human errors, at-risk behaviors, and reckless conduct, ultimately fostering a culture of safety and accountability.
Regulatory experts stress that effective training and validation processes are crucial for ensuring accurate classification of adverse events. By prioritizing these initiatives, entities can enhance patient safety outcomes and maintain adherence to regulatory frameworks. This proactive approach transforms their handling of adverse events into a robust system that supports systemic improvements.
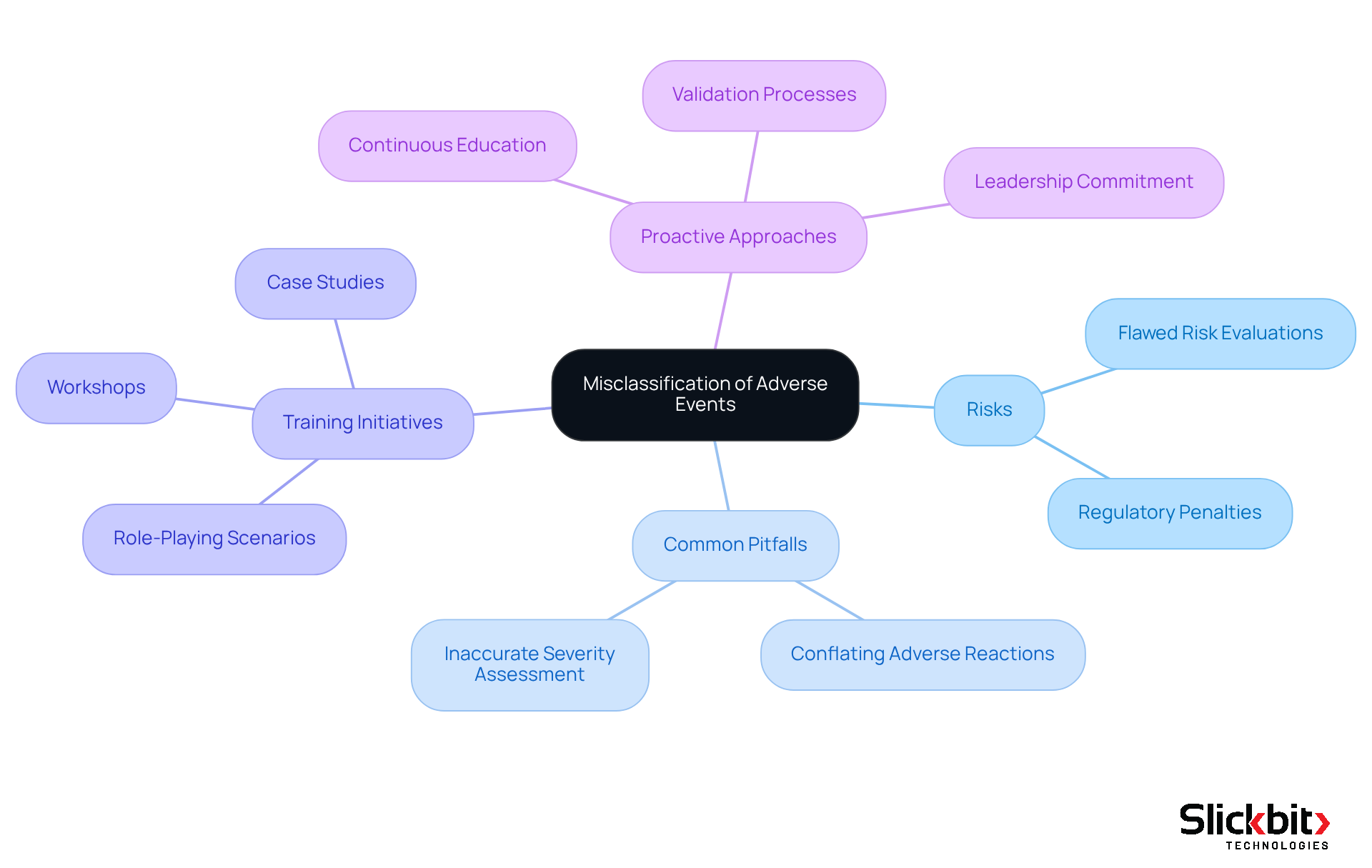
Incomplete or Missing Data: Strategies for Comprehensive Reporting
To address the pressing challenges posed by incomplete or absent information in ICSR, organizations must implement several effective strategies, particularly through the utilization of AI technologies. The integration of digital templates featuring mandatory fields is essential, as it guarantees that all crucial information is captured from the outset. This approach has been shown to significantly enhance information completeness.
Furthermore, AI-powered automated reminders for reporters can facilitate timely information submission, thereby reducing the likelihood of overlooked details. Routine evaluations of submitted documents, augmented by AI analysis, are equally vital; they help identify recurring trends of missing data, enabling organizations to refine their data collection methods and elevate the overall quality of submissions.
By adopting these strategies, including insights from AI applications in FDA inspections, companies can transform their reporting practices to improve ICSR, ultimately bolstering compliance and enhancing patient safety.
Missed Regulatory Deadlines: Ensuring Timely ICSR Submissions
Missed regulatory deadlines can lead to significant consequences, including hefty fines and reputational damage for organizations. Over 1,000,000 reports were not demonstrably reported within the timeframe allowed by the FDA, underscoring the critical need for timely ICSR submissions. To mitigate these risks, companies must create clear timelines for disclosures based on the severity of adverse events.
Implementing robust case tracking systems can effectively monitor submission deadlines and provide alerts for upcoming due dates, ensuring that no critical reports are overlooked. Furthermore, utilizing AI technologies, such as those applied in Trend 483, can enhance compliance by recognizing patterns in systemic risks and recurring violations, thus guiding improved documentation practices.
Fostering a culture of responsibility within the organization is crucial, as it motivates teams to prioritize prompt submission and adhere to regulatory requirements related to ICSR. As compliance specialist Alivia Kaylor emphasizes, a proactive strategy involving robust internal training and frequent audits can significantly improve the precision and timeliness of regulatory submissions. Additionally, employing automation can streamline the documentation process, ultimately safeguarding patient safety and upholding corporate integrity.
Poor Data Quality from Non-Traditional Sources: Mitigation Techniques
Non-traditional information sources, such as social media and electronic health records (EHRs), present significant challenges in maintaining quality for ICSR reporting. To effectively address these challenges, organizations must establish robust validation protocols that rigorously evaluate the reliability of incoming information. Furthermore, employing AI-driven analytics can significantly assist in identifying and eliminating low-quality information, ensuring that only precise and pertinent content is included in reports. Additionally, training personnel on the nuances of information from diverse sources can enhance the overall quality of reporting. By implementing these strategies, organizations can improve the integrity and reliability of their ICSR submissions.
Inadequate Training and Technology Gaps: Building Competence in ICSR Reporting
To effectively address the challenges posed by insufficient training and technology gaps in ICSR, organizations must prioritize investment in comprehensive training programs. Slickbit offers personalized AI training sessions and practical workshops tailored for pharmaceutical R&D teams, emphasizing regulatory requirements, data management practices, and the application of advanced analytics technologies.
Regular workshops are crucial for keeping staff updated on best practices and emerging technologies. Furthermore, fostering a culture of continuous learning empowers employees to pursue new knowledge and skills, significantly enhancing the quality of submissions for ICSR.
Industry leaders assert that closing these technology gaps is essential for ensuring compliance and enhancing overall efficiency, ultimately safeguarding patient safety. By leveraging Slickbit's AI solutions, organizations can further streamline these processes, ensuring they remain at the forefront of compliance and operational excellence.
Overlooking Regional Regulatory Nuances: Navigating Local Compliance
Overlooking regional regulatory details can significantly jeopardize adherence in safety-related documentation. Organizations must remain vigilant regarding the specific requirements in each jurisdiction, including submission timelines, data formatting, and documentation standards. Establishing a centralized compliance team dedicated to monitoring regulatory changes is essential. This group can provide continuous support to local teams, ensuring that all submissions conform to regional requirements. For instance, the FDA's recent acceptance of the E2B(R3) format underscores the necessity for timely adaptation to evolving standards.
Furthermore, as pharmaceutical companies expand globally, they encounter the challenge of navigating diverse regulations, which can heighten the risk of non-compliance and jeopardize patient safety. Compliance specialists emphasize that a proactive strategy, incorporating frequent training and updates on local regulations, is crucial for maintaining adherence to requirements. By fostering a culture of compliance and leveraging local expertise, organizations can enhance their accuracy in disclosures and protect their business reputation.
Summary of Common Pitfalls in ICSR Reporting: Key Takeaways
ICSR poses significant challenges that can profoundly affect regulatory compliance. One of the most common pitfalls is the misclassification of adverse events, which distorts safety signals and leads to skewed analyses. In addition, incomplete or absent information poses a major issue; studies indicate that one in three reports from patients lacks essential details, increasing the risk of rejection by regulators. Furthermore, missed regulatory deadlines can have serious consequences, with nearly a third of reports submitted late, often due to manual workflows and high volumes.
Poor data quality, particularly from non-traditional sources such as social media and electronic health records, complicates the landscape further. Notably, patient-reported ICSR cases exhibit 24.88 times higher odds of misclassification compared to those from pharmaceutical companies. Insufficient training on reporting standards and neglecting regional regulatory specifics exacerbate these challenges, leading to costly errors that delay regulatory approvals and jeopardize patient safety. Organizations that proactively identify and address these issues can significantly enhance the accuracy and compliance of their submission reports, ultimately fostering a more robust pharmacovigilance framework.
Recommendations for Avoiding ICSR Reporting Pitfalls
To effectively navigate the complexities of ICSR reporting and avoid common pitfalls, organizations should adopt the following strategies:
-
Utilize AI-Driven Tools: Implementing AI technologies for information validation and classification can significantly enhance data precision. AI algorithms extract essential information from narratives, including patient demographics and adverse reactions, thereby improving overall quality.
-
Establish Clear Reporting Timelines: Setting definitive deadlines for submissions and actively monitoring adherence is crucial. A study revealed that the proportion of ICSR reported within 30 days improved from 10% to 47% after the implementation of structured timelines, underscoring the effectiveness of this approach.
-
Invest in Comprehensive Training Programs: Equipping staff with the necessary skills to utilize AI tools effectively is essential. Training programs should focus on the integration of AI in pharmacovigilance, ensuring that teams are adept at leveraging these technologies for enhanced documentation practices.
-
Regularly auditing the quality and completeness of ICSR data is vital. Research indicates that thoroughness in documentation increased from 44% to 80% following systematic quality control interventions, highlighting the importance of continuous assessment.
-
Stay Informed About Regulatory Requirements: Keeping abreast of regional regulatory changes and adapting processes accordingly is imperative. As AI regulations evolve, organizations must ensure their AI systems comply with the latest standards to avoid potential penalties and maintain operational integrity.
By adopting these strategies, companies can significantly enhance their sustainability disclosure methods, ensuring compliance with regulatory standards while improving overall efficiency.
Continuous Improvement in ICSR Reporting: Embracing Change
To achieve continuous improvement in ICSR documentation, organizations must proactively embrace change and adapt to evolving technologies and regulatory demands. This requires:
- A consistent evaluation and enhancement of documentation processes
- Investment in cutting-edge technologies such as AI and automation
- The cultivation of an innovative culture
For example, a prominent pharmaceutical company effectively standardized its safety surveillance across various units by implementing a signal detection platform, which reduced time-to-benefit and increased operational efficiency.
Furthermore, the FDA's recent shift to daily releases of adverse event information through the FDA Adverse Event Reporting System (FAERS) underscores the imperative for organizations to update their practices to enhance transparency and responsiveness.
Industry leaders emphasize that the adoption of technology not only improves accuracy in documentation but also streamlines processes, ultimately leading to better patient safety outcomes. By prioritizing these strategies, organizations can significantly bolster their compliance and efficiency in ICSR.
Conclusion
To navigate the complexities of Individual Case Safety Reports (ICSR) and avoid common pitfalls, organizations must prioritize a proactive and informed approach. Leveraging AI-driven solutions, investing in comprehensive training, and maintaining awareness of regulatory requirements are crucial strategies for enhancing reporting accuracy and compliance. By adopting these measures, organizations can significantly mitigate risks associated with misclassification, incomplete data, and missed deadlines, ultimately safeguarding patient safety and maintaining regulatory integrity.
Key insights from the article underscore the importance of understanding the nuances of adverse event reporting. The role of technology in improving data quality and the need for continuous improvement in documentation practices are paramount. Organizations are encouraged to:
- Implement structured timelines for submissions
- Conduct regular audits
- Foster a culture of learning and innovation
These initiatives not only enhance the quality of ICSR submissions but also support a robust pharmacovigilance framework.
In conclusion, the significance of addressing ICSR reporting pitfalls cannot be overstated. By embracing change and employing advanced technologies, organizations can streamline their processes and ensure compliance with evolving regulatory standards. The commitment to continuous improvement and proactive risk management is essential for achieving excellence in ICSR reporting, ultimately leading to better patient outcomes and enhanced organizational reputation.
Frequently Asked Questions
What is Slickbit and how does it enhance ICSR reporting accuracy?
Slickbit.ai develops AI-driven solutions that enhance the accuracy of Individual Case Safety Reports (ICSR) by leveraging advanced analytics and machine learning to optimize documentation processes and ensure compliance with regulatory standards.
What specific AI solutions does Slickbit offer to improve ICSR?
One notable solution is the AI-driven regulatory intelligence assistant Lumino, which provides precise responses from FDA guidance documents, improving the accuracy of ICSR submissions. Additionally, automation tools like Vault Redact help identify and remove sensitive information to bolster compliance.
What are the risks associated with using AI in pharmacovigilance?
A significant risk is the 'black-box' nature of AI models, which can complicate transparency and accountability in pharmacovigilance, potentially affecting the reliability of safety data submissions.
What are the common pitfalls in the classification of adverse events?
Common pitfalls include conflating adverse reactions with unrelated medical conditions and inaccurately assessing the severity of incidents, which can lead to flawed risk evaluations and regulatory penalties.
What are the consequences of misclassifying adverse events?
Misclassifying adverse events can undermine the integrity of safety documentation systems, potentially leading to patient safety threats and regulatory compliance issues.
How can organizations improve the classification of adverse events?
Organizations can improve classification by developing comprehensive training initiatives that include role-playing scenarios, workshops, and case studies focused on negative event documentation and just culture principles.
What strategies can be implemented to address incomplete or missing data in ICSR?
Effective strategies include using digital templates with mandatory fields to capture crucial information, employing AI-powered automated reminders for timely submissions, and conducting routine evaluations of submitted documents to identify trends in missing data.
How can AI technologies enhance the overall quality of ICSR submissions?
AI technologies can improve submission quality by ensuring completeness through mandatory fields, facilitating timely information submission with reminders, and analyzing submitted documents to refine data collection methods.