Overview
The article underscores the critical importance of understanding the full form of the Trial Master File (TMF) for pharmaceutical R&D managers, emphasizing its pivotal role in the realm of clinical trials. A well-structured TMF is not merely beneficial; it is essential for ensuring regulatory compliance and maintaining the integrity of trials. Furthermore, best practices, alongside technological advancements such as artificial intelligence and electronic TMFs (eTMFs), play vital roles in enhancing both efficiency and accuracy in TMF management. By implementing these strategies, R&D managers can significantly improve their operational effectiveness, thereby fostering a more reliable clinical trial process.
Introduction
The management of Trial Master Files (TMFs) represents a pivotal challenge in pharmaceutical research, where many R&D managers encounter significant complexities. Understanding the full form of TMF is essential; it embodies a comprehensive array of documentation that ensures compliance and upholds the integrity of clinical trials. This article explores crucial insights into the transformative impact of AI and technology in optimizing TMF processes, addressing the challenges faced by professionals in this domain. How can R&D managers harness these advancements to enhance efficiency and achieve inspection readiness amid a continuously evolving regulatory landscape?
Slickbit: AI Solutions for Optimizing Trial Master File Management
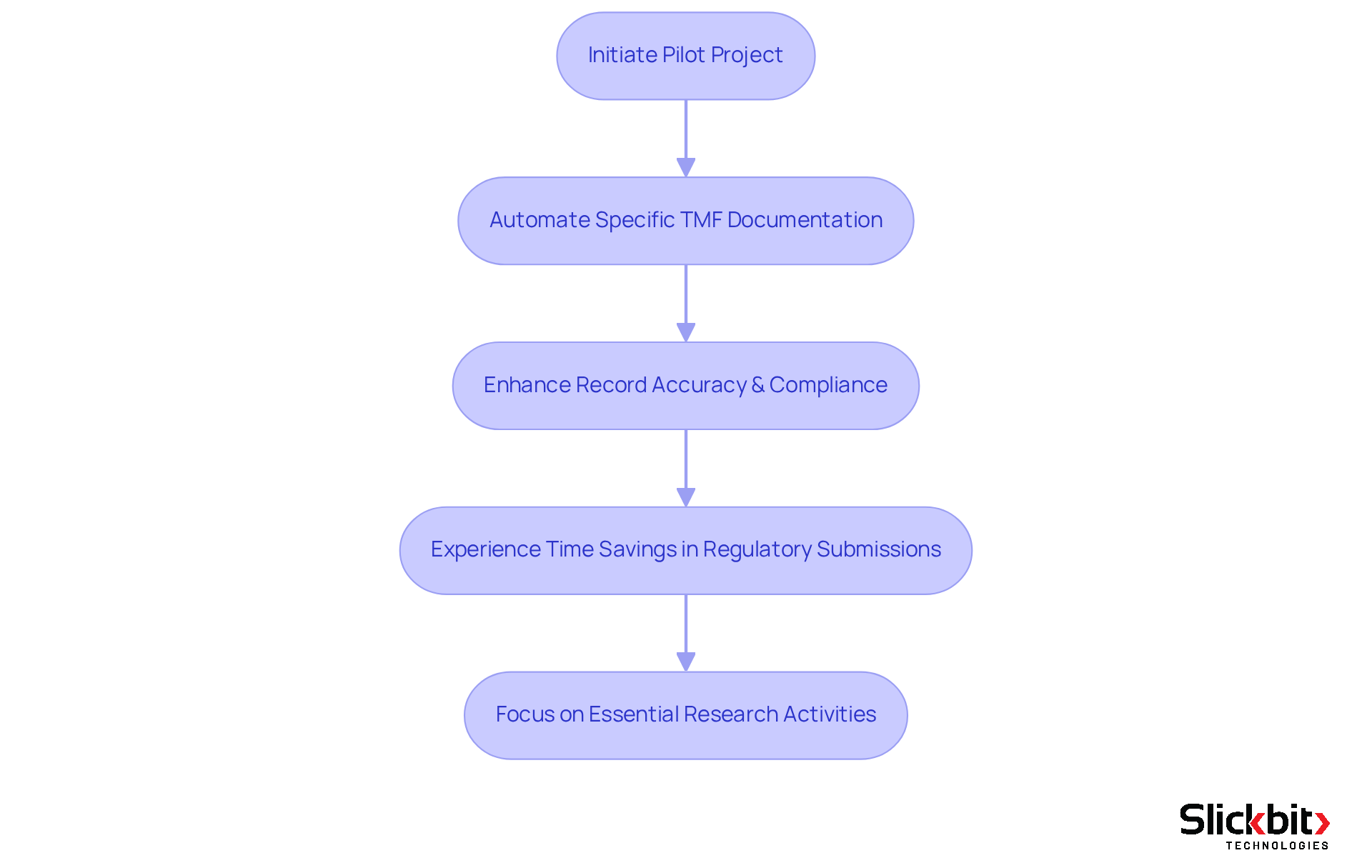
Slickbit.ai provides advanced AI solutions that significantly improve the management of Trial Master Files (TMFs), which is the TMF full form, for pharmaceutical companies. By leveraging AI technologies, R&D managers can automate routine record-keeping tasks related to the TMF full form, ensuring compliance and enhancing the accuracy of records. This automation not only expedites the clinical trial process but also reduces the risk of errors, enabling teams to focus on essential research activities.
Successful implementations of AI in TMF records have shown considerable time savings, with regulatory submission activities experiencing up to a 63% reduction in processing time. Furthermore, AI-powered tools such as Lumino and Vault Redact enhance record accuracy and regulatory compliance, which are critical for achieving successful trial outcomes.
Expert insights underscore the importance of AI in revolutionizing TMF oversight, particularly in relation to the TMF full form, highlighting its role in promoting efficiency and reliability in clinical trials. For R&D managers looking to explore AI solutions for TMF oversight, initiating a pilot project that focuses on automating a specific aspect of TMF documentation could serve as a practical first step.
Understanding the Trial Master File (TMF) and Its Importance in Clinical Trials
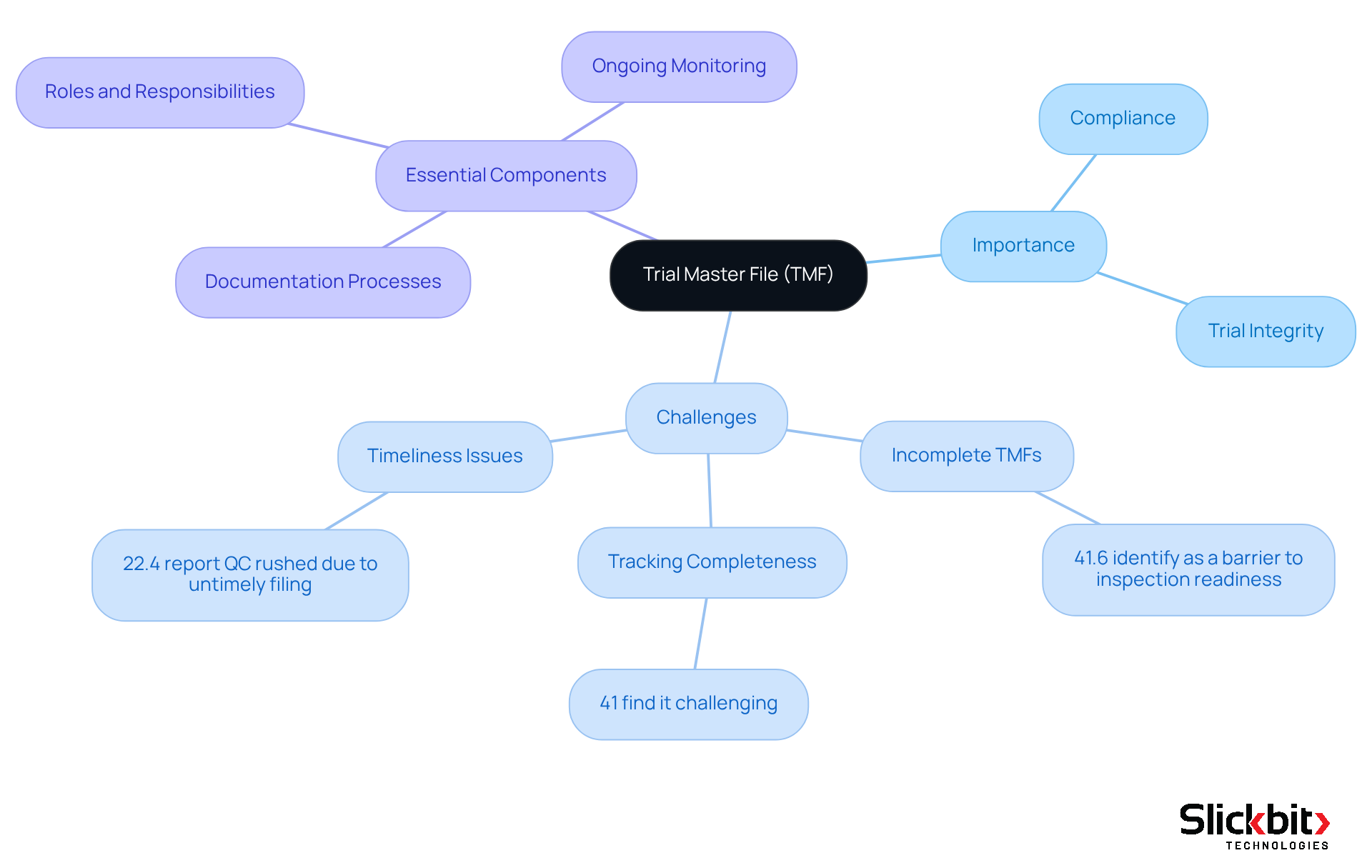
The TMF full form, which stands for Trial Master File, serves as a crucial repository of documents that supports the execution of clinical trials. It functions as a comprehensive record that encompasses the planning, execution, and outcomes of the trial, thereby ensuring compliance with all regulatory requirements.
For R&D managers, understanding the TMF full form's structure and contents is imperative, as it significantly impacts the trial's integrity and adherence to regulatory standards. Notably, 41.6% of professionals identify incomplete TMFs as a major barrier to achieving inspection readiness, underscoring the critical need for diligent TMF oversight and clarity on the TMF full form.
Furthermore, 41% of TMF professionals regard completeness as the most challenging metric to monitor, which highlights the complexities associated with TMF management, including understanding the TMF full form. The TMF full form should be viewed not merely as a collection of documents, but rather as a valuable data source that provides insights into the quality of clinical trials.
Essential components of an effective TMF full form include:
- Well-defined documentation processes
- Clearly outlined roles and responsibilities
- Ongoing monitoring of activities related to the TMF full form
In fact, 17.8% of respondents indicated that the absence of a well-defined process presents a challenge to timely filing within the TMF full form, highlighting the necessity for structured methodologies. Additionally, 40% of respondents acknowledged enhanced efficiency as a significant advantage of implementing an eTMF system, which can streamline the TMF full form management process.
By fostering a robust culture around the TMF full form, organizations can enhance compliance and optimize their trial processes, ultimately leading to improved outcomes in pharmaceutical research and development.
Key Components of a Trial Master File: Structure and Essential Documents
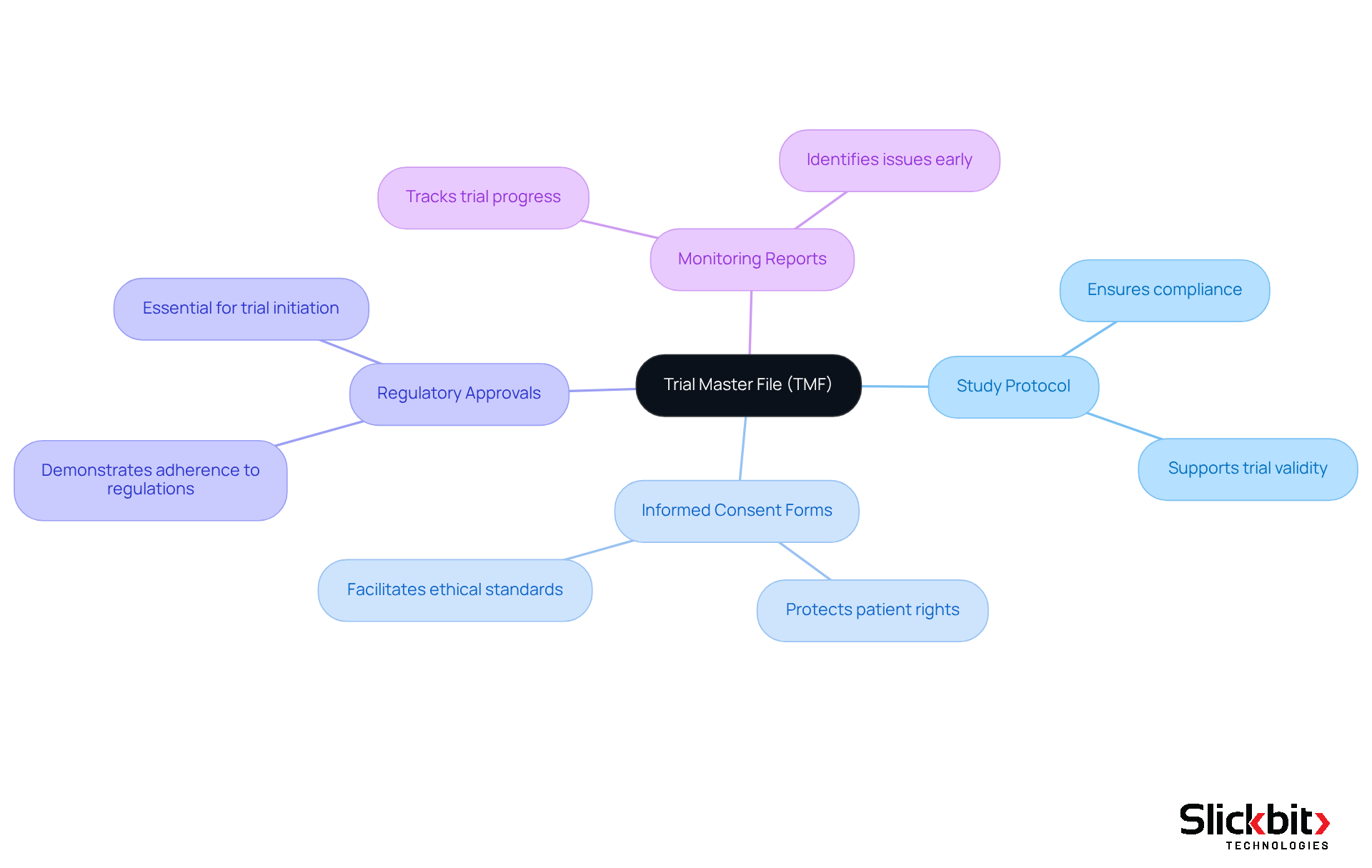
The TMF full form refers to a well-structured Trial Master File that is essential for the success of clinical trials, comprising several key components, including:
- The study protocol
- Informed consent forms
- Regulatory approvals
- Monitoring reports
Each document is critical in demonstrating adherence to regulatory standards and supporting the trial's validity. R&D managers must ensure that these documents are not only present but also meticulously organized to facilitate easy access and review during inspections.
Furthermore, leveraging Slickbit.ai's AI-powered Regulatory Intelligence allows Lumino to enhance the accuracy of responses derived from FDA and global guidance documents, streamlining the retrieval of essential information. In addition, Vault Redact automates the identification and removal of sensitive information, ensuring compliance and safeguarding patient privacy throughout the documentation process.
The Rise of Electronic Trial Master Files (eTMFs) in Pharma R&D
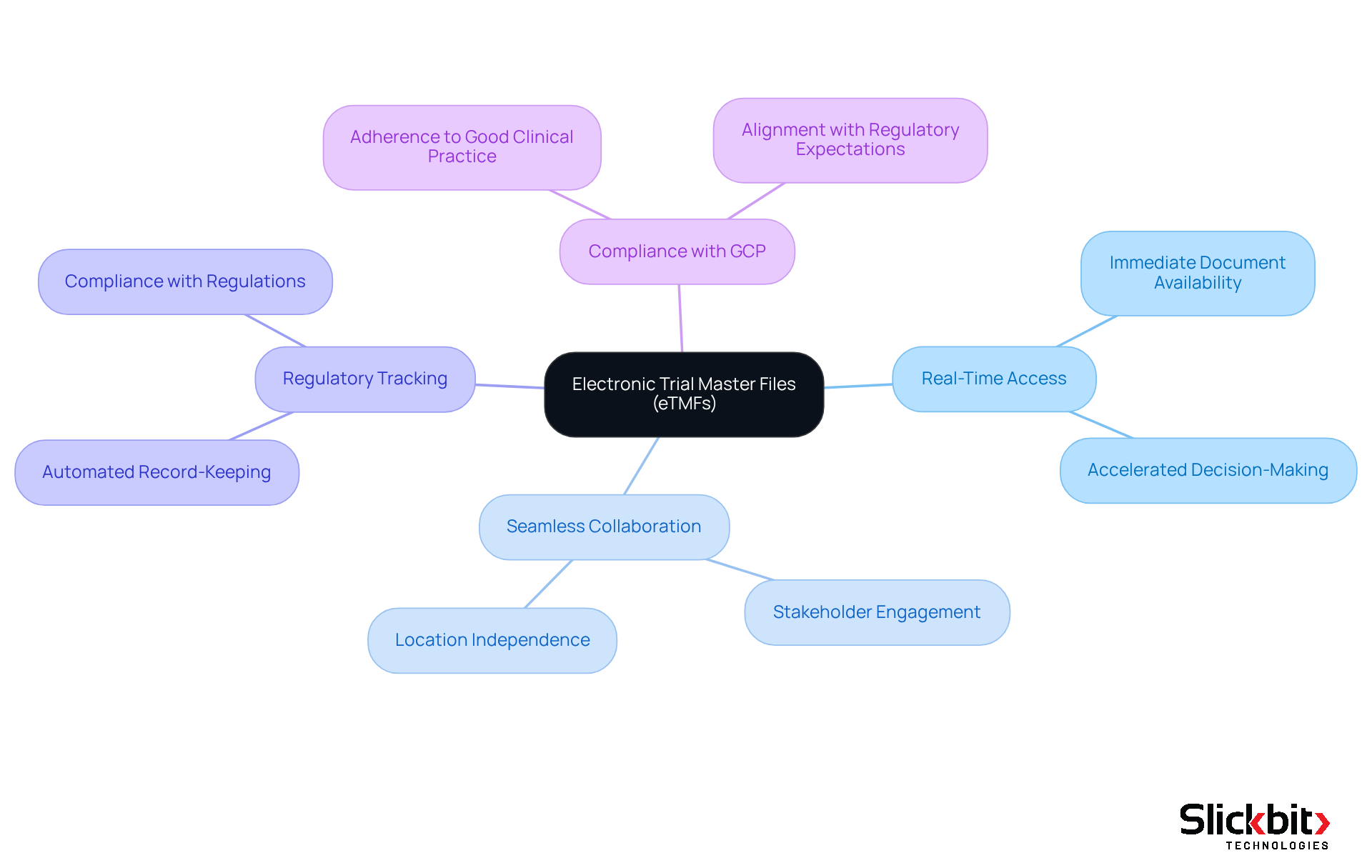
The adoption of Electronic Trial Master Files (eTMFs) has significantly transformed clinical trial oversight, offering substantial benefits that enhance operational efficiency. eTMFs provide real-time access to essential documents, enabling stakeholders to collaborate seamlessly, irrespective of their location. This immediate access not only accelerates decision-making but also cultivates a more integrated approach to trial management.
Furthermore, eTMFs are pivotal in regulatory tracking. By automating the record-keeping process and ensuring compliance with all regulatory requirements, eTMFs assist organizations in adhering to Good Clinical Practice (GCP) principles. A case study featuring IQVIA exemplifies this, demonstrating how insights from adherence position papers have been instrumental in aligning their product roadmap with regulatory expectations, ultimately enhancing their trial oversight capabilities.
As R&D leaders increasingly embrace digital solutions, eTMFs are establishing themselves as the benchmark for efficient trial record handling, driving advancements in collaboration and adherence throughout the industry.
Regulatory Compliance and the Trial Master File: What R&D Managers Must Know
Regulatory adherence is fundamental to effective management of the TMF full form. R&D managers must grasp the specific requirements set by regulatory agencies such as the FDA and EMA, which govern the TMF full form of record-keeping practices. Key aspects include:
- Timelines for Document Submission: Understanding the timelines for submitting TMF documents is crucial. For instance, the FDA mandates that essential documents be submitted within specific timeframes to ensure compliance and facilitate timely reviews.
- Accurate Record Maintenance: Maintaining precise and complete records is non-negotiable. Incomplete documentation can lead to significant regulatory issues, with 41.6% of TMF teams reporting challenges in this area, jeopardizing inspection readiness, which emphasizes the importance of understanding the tmf full form.
Regular audits are essential for ensuring that the TMF full form, or trial master files, remain compliant with evolving regulations. This proactive approach aids in identifying potential gaps and facilitates timely corrective actions.
R&D managers can enhance adherence to the TMF full form by integrating these practices into their workflows. For example, employing automation tools like Trend 483, which utilizes AI to analyze FDA inspection trends, can optimize TMF oversight through document upload and classification, ensuring that all submissions align with FDA and EMA guidelines. Furthermore, fostering a robust TMF culture within teams, supported by knowledgeable experts, can proactively address regulatory challenges, ultimately improving study outcomes. This approach mirrors the operational efficiencies observed in hospitality reservation management, where AI systems enhance adherence and streamline processes.
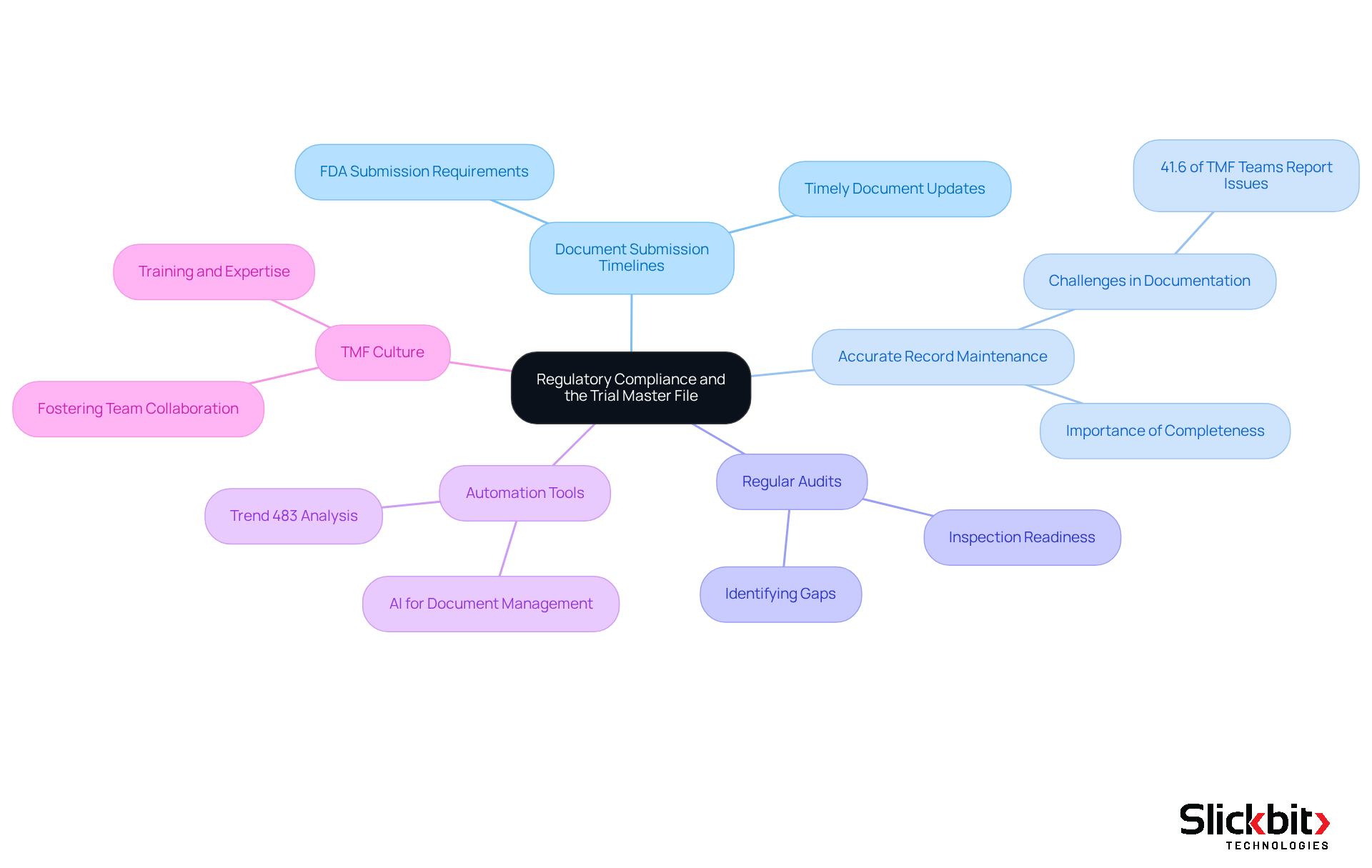
Best Practices for Ensuring TMF Quality and Inspection Readiness
To ensure TMF quality and inspection readiness, R&D managers must adopt best practices, including:
- Understanding the TMF full form
- Conducting regular document reviews
- Implementing a clear version control system
- Performing mock inspections
Furthermore, utilizing AI tools like Trend 483 can significantly enhance adherence by identifying systemic risks and recurring violations from FDA inspections. Trend 483 empowers users to search, filter, and view full 483s, offering deeper insights into potential issues.
In addition, training team members on TMF requirements and utilizing checklists can streamline processes and ensure that all necessary documents are meticulously organized. By integrating AI-driven insights, R&D managers can not only improve operational efficiency but also maintain a proactive approach to compliance.
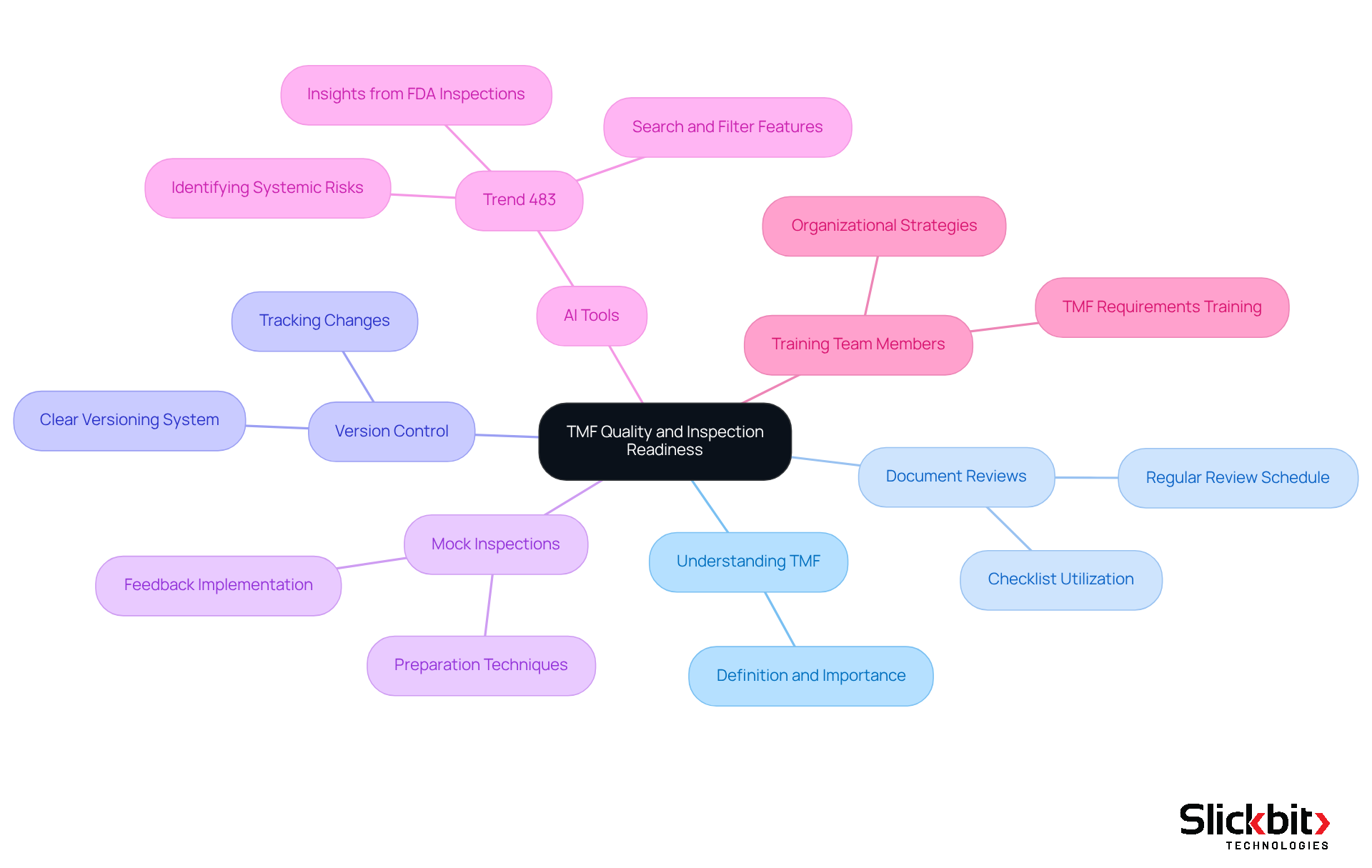
Collaborating with CROs: Effective Management of Trial Master Files
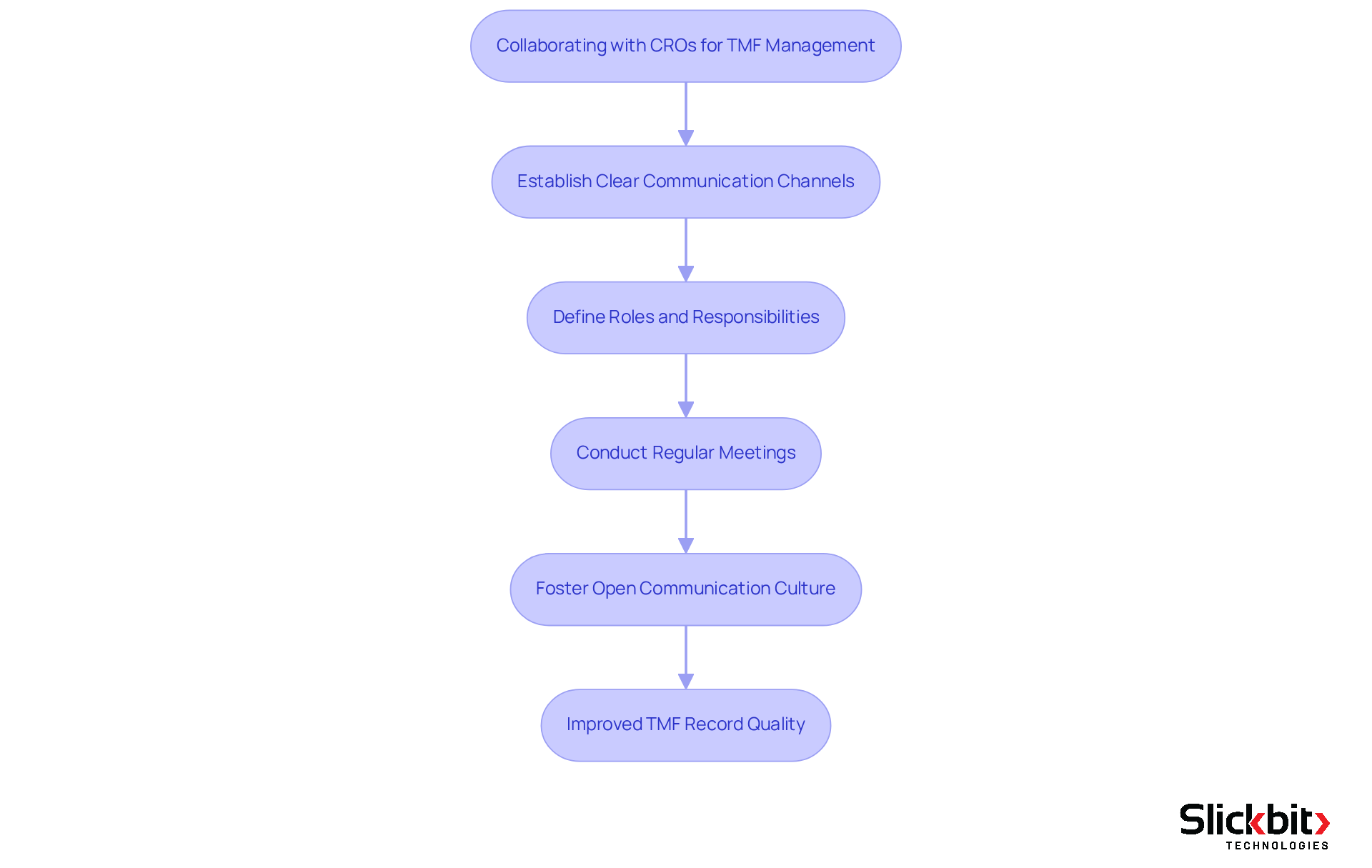
Collaboration with Contract Research Organizations (CROs) is essential for effective Trial Master File (TMF) management, which underscores the importance of knowing the TMF full form, as these entities play a significant role in trial execution. R&D managers must prioritize the establishment of clear communication channels and the definition of roles and responsibilities related to the TMF full form records. Regular meetings and updates are crucial to ensuring alignment among all stakeholders, thereby facilitating a comprehensive and current understanding of the TMF full form.
Statistics indicate that effective communication can enhance accuracy in records, with organized records improving the quality score from 64.35 to 77.2. By fostering a culture of open communication and accountability, organizations can mitigate risks associated with record-keeping gaps and ensure compliance with regulatory standards. As Dawn Niccum emphasizes, 'Proper record-keeping and proactive risk oversight are essential for effective sponsor supervision.'
Implementing organized communication strategies can lead to significant improvements in the quality of TMF records, which is essential for understanding the TMF full form and ensuring that all records are both comprehensive and compliant.
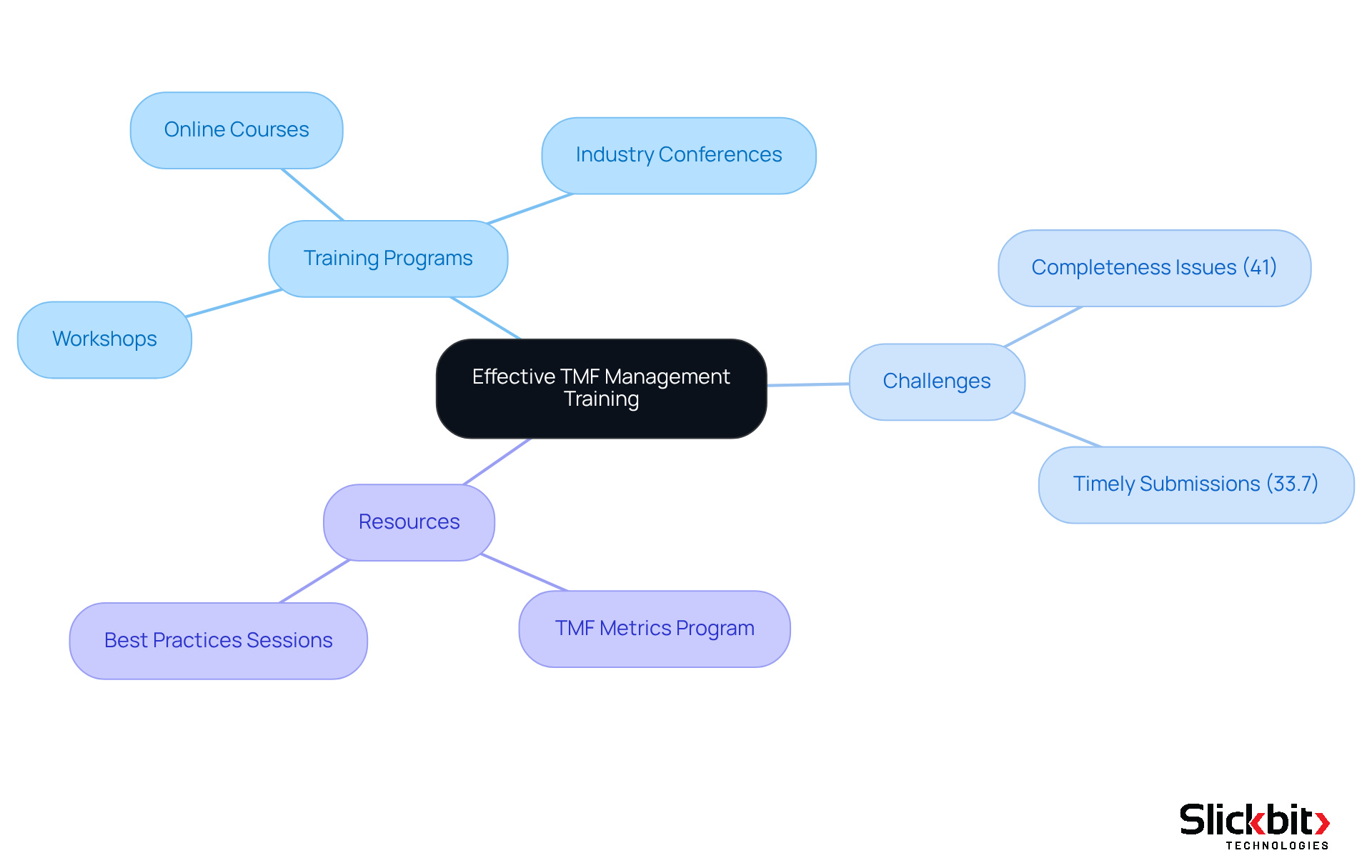
Training and Resources for Effective Trial Master File Management
To enhance TMF oversight capabilities, R&D managers must prioritize training programs that cover the TMF full form, the fundamentals of TMF records, regulatory requirements, and best practices. Engaging in workshops, online courses, and industry conferences can yield critical insights and ensure teams remain informed about the latest trends and technologies in TMF management, particularly the TMF full form.
Recent surveys reveal that approximately 41% of TMF professionals encounter challenges related to completeness, while 33.7% struggle with timely document submissions. This underscores the urgent need for robust training initiatives. Workshops emphasizing the practical applications of TMF processes can markedly elevate team awareness and engagement, fostering a culture that values timely and accurate documentation.
Furthermore, industry conferences frequently feature sessions dedicated to TMF best practices, providing R&D managers with opportunities to learn from experts and network with peers. Investing in these resources not only enhances individual skills but also reinforces overall organizational adherence and efficiency in managing trial master files, which is known by the TMF full form.
Moreover, establishing a TMF metrics program is essential for identifying gaps and improving TMF practices, ensuring that teams are well-equipped to address the challenges posed by contemporary clinical trials.
Leveraging Technology to Enhance Trial Master File Processes
Leveraging technology, such as AI and automation tools, presents a significant opportunity to enhance processes related to the TMF full form. R&D managers are encouraged to explore innovative solutions like Slickbit's Lumino, an AI-powered Regulatory Intelligence assistant that delivers accurate, traceable answers from FDA and global guidance documents. Additionally, Vault Redact automates the identification and removal of PII and PHI from documents. These tools not only foster collaboration and oversight tracking but also improve efficiency and reduce the likelihood of errors, ensuring that the TMF full form is always inspection-ready.
Future Trends in Trial Master File Management for Pharma R&D
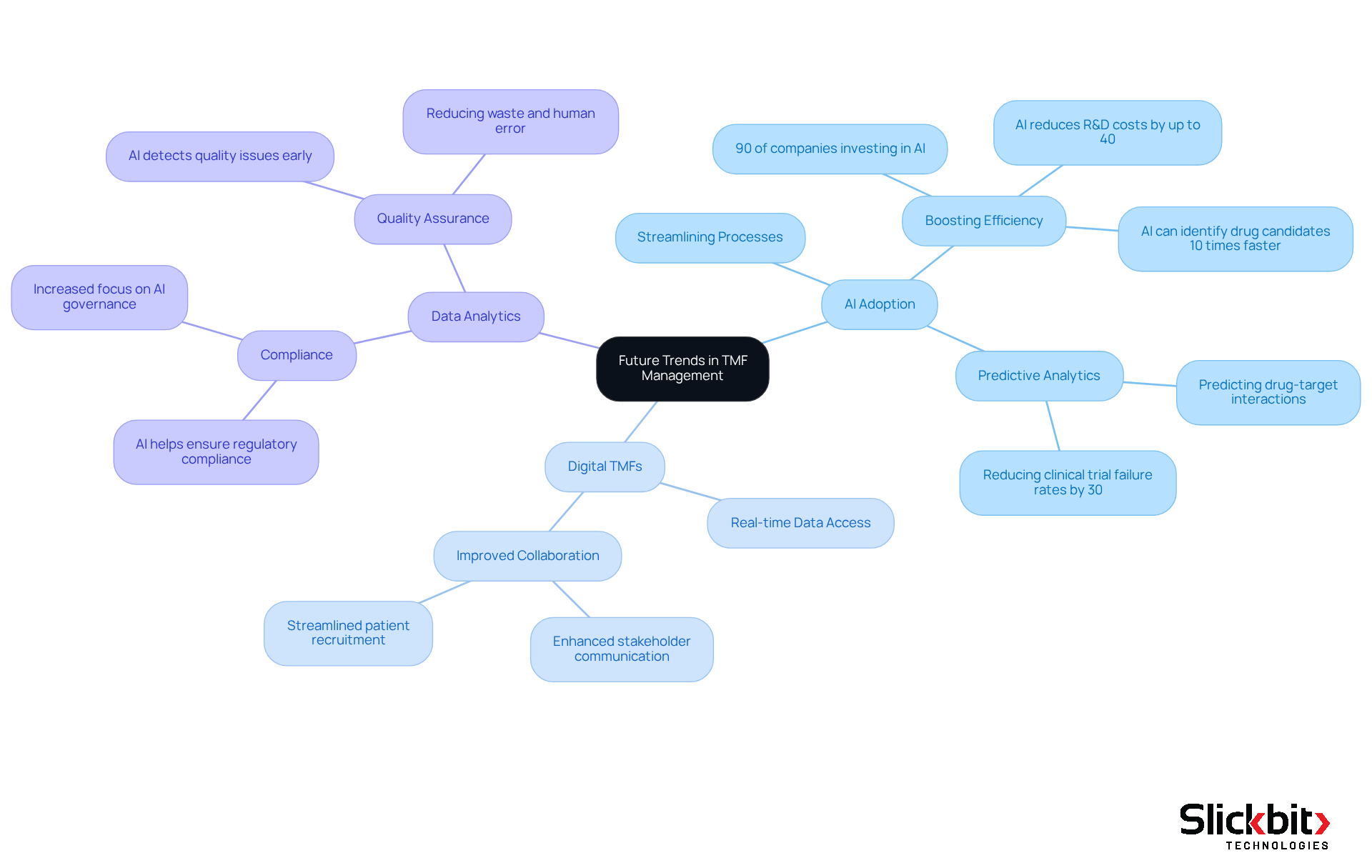
The pharmaceutical industry is experiencing transformative changes that are redefining the management of the TMF full form. A prominent trend is the increasing adoption of AI-driven solutions, revolutionizing how R&D teams manage data and navigate regulations. Notably, statistics show that over 90% of pharmaceutical companies are investing in AI-driven drug discovery, acknowledging its capacity to streamline processes and boost efficiency. This evolution is further supported by the shift towards fully digital electronic TMFs (eTMFs), which enable real-time data access and foster improved collaboration among stakeholders.
Furthermore, the focus on data analytics is becoming vital for ensuring compliance and quality assurance. AI technologies possess the ability to analyze extensive datasets, predicting potential issues before they escalate, thereby reducing waste and minimizing human error. As R&D managers adapt to these advancements, it is crucial to remain informed about these trends to refine strategies and harness new technologies that enhance the TMF full form management. The future of TMF processes is set to be more efficient, compliant, and data-driven, ultimately resulting in expedited drug development timelines and enhanced patient outcomes.
Conclusion
The exploration of the Trial Master File (TMF) full form reveals its critical role in enhancing the efficiency and compliance of pharmaceutical research and development. Understanding the TMF transcends mere recognition as a collection of documents; it embodies a commitment to maintaining the integrity of clinical trials. By leveraging advanced technologies and adopting best practices, R&D managers can ensure their TMF processes are streamlined, compliant, and prepared for regulatory scrutiny.
Key insights from the article highlight the transformative impact of AI and electronic TMFs (eTMFs) on trial management. The integration of AI tools not only accelerates document processing but also enhances accuracy and compliance, ultimately leading to more successful trial outcomes. Furthermore, fostering effective collaboration with Contract Research Organizations (CROs) and investing in comprehensive training programs are essential steps toward optimizing TMF management.
In light of these insights, R&D managers are encouraged to embrace the evolving landscape of TMF management by adopting innovative technologies and practices. As the pharmaceutical industry progresses, staying informed and proactive in TMF oversight will be paramount for achieving regulatory compliance and enhancing the quality of clinical trials. Embracing these changes will not only streamline processes but also contribute to improved patient outcomes and expedited drug development timelines.
Frequently Asked Questions
What is the Trial Master File (TMF)?
The Trial Master File (TMF) is a crucial repository of documents that supports the execution of clinical trials, encompassing the planning, execution, and outcomes of the trial to ensure compliance with regulatory requirements.
Why is the TMF important for clinical trials?
The TMF is important because it ensures the integrity of the trial and adherence to regulatory standards. Incomplete TMFs are a major barrier to achieving inspection readiness, highlighting the need for diligent oversight.
What are the key components of a TMF?
Key components of a TMF include the study protocol, informed consent forms, regulatory approvals, and monitoring reports. These documents are essential for demonstrating adherence to regulatory standards.
How can AI solutions improve TMF management?
AI solutions can automate routine record-keeping tasks related to TMF management, ensuring compliance, enhancing record accuracy, and expediting the clinical trial process while reducing the risk of errors.
What are some benefits of using AI in TMF management?
Successful implementations of AI in TMF management have shown up to a 63% reduction in processing time for regulatory submissions, improved record accuracy, and enhanced regulatory compliance.
What challenges do R&D managers face regarding TMF completeness?
R&D managers face challenges such as monitoring the completeness of TMFs, with 41% of professionals identifying it as the most challenging metric. Incomplete TMFs can hinder inspection readiness.
What steps can R&D managers take to optimize TMF management?
R&D managers can initiate pilot projects focusing on automating specific aspects of TMF documentation and foster a robust culture around TMF management to enhance compliance and optimize trial processes.
How does Slickbit.ai support TMF management?
Slickbit.ai provides AI-powered tools like Lumino and Vault Redact, which enhance record accuracy, automate sensitive information removal, and streamline the retrieval of essential regulatory information.




