Overview
The article titled "10 Key Insights on MDR Full Form in Medical Device Reporting" provides a comprehensive overview of the crucial elements of Medical Device Reporting (MDR) and its significant implications for compliance within the healthcare sector. It underscores the necessity for manufacturers and importers to grasp MDR's requirements to uphold patient safety and adhere to regulatory standards. Furthermore, the article delineates specific obligations, reporting timelines, and the critical role of accurate documentation in enhancing the overall safety of healthcare equipment. This understanding is not merely beneficial; it is essential for navigating the complexities of medical device regulations effectively.
Introduction
Understanding the complexities of medical device reporting is paramount in an industry where compliance can significantly impact patient safety and regulatory adherence. This article explores the multifaceted realm of Medical Device Reporting (MDR), presenting ten critical insights that underscore the necessity of precise documentation and prompt reporting. As organizations increasingly adopt AI solutions to bolster compliance, a pivotal question emerges: how can manufacturers and healthcare professionals adeptly navigate the intricate landscape of MDR while fulfilling stringent regulatory requirements? By examining these insights, stakeholders can enhance their understanding and effectively address the challenges posed by MDR.
Slickbit: AI Solutions for Enhancing Medical Device Reporting Compliance
Slickbit.ai delivers advanced AI solutions that significantly enhance adherence in healthcare equipment documentation. By leveraging AI-driven analytics, organizations can automate documentation processes, ensuring both accuracy and compliance with stringent regulatory requirements. This integration not only streamlines workflows but also reduces the risk of errors, which is vital for upholding regulatory standards.
As emphasized by the FDA, the incorporation of AI in healthcare applications mandates strict compliance with regulations like the mdr full form in medical, underscoring the importance of reliable and precise documentation in the healthcare sector. Furthermore, a remarkable 96% of surveyed leaders believe AI can enhance patient outcomes and experiences, showcasing the transformative potential of AI in improving compliance.
Slickbit's AI-powered Regulatory Intelligence assistant, Lumino, enables pharma teams to access accurate, traceable answers from FDA and global guidance documents. Meanwhile, Vault Redact automates the identification and removal of PII and PHI from documents, ensuring secure content management.
With our rapid MVP development, Life Sciences companies can swiftly implement these solutions, drastically reducing the time needed to achieve compliance. This efficiency not only accelerates documentation workflows but also allows organizations to focus on innovation while ensuring adherence to all necessary regulations.
Understanding Medical Devices: Definitions Under MDR
Under the mdr full form in medical, a medical device encompasses any instrument, apparatus, appliance, software, implant, reagent, or material intended for medical purposes. This comprehensive definition includes a diverse array of products, ranging from simple bandages to sophisticated software utilized in diagnostics.
Understanding these definitions is essential for producers and importers, as it enables them to identify which products fall under the mdr full form in medical's jurisdiction and require appropriate documentation. Consequently, grasping the nuances of this definition is not merely academic; it is a critical step towards ensuring compliance and operational success in the medical device landscape.
Mandatory Medical Device Reporting Requirements: What You Must Know
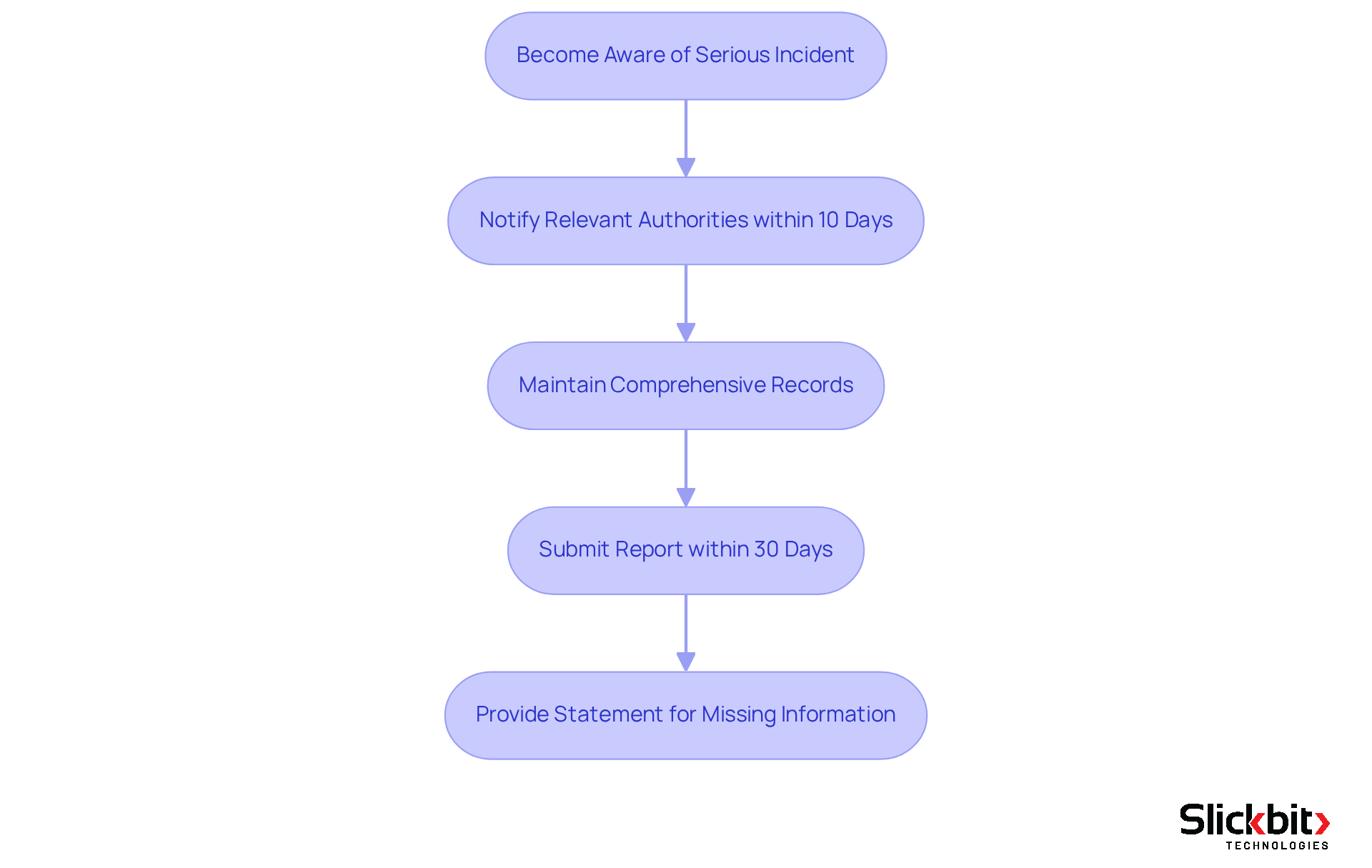
Under the Medical Device Regulation (MDR), manufacturers are mandated to report serious incidents and field corrective actions without delay. Specifically, they must notify relevant authorities within 10 days of becoming aware of a serious incident, which encompasses any device-related death or serious injury. This requirement underscores the critical nature of prompt documentation in safeguarding patient well-being. Furthermore, manufacturers are obliged to maintain comprehensive records of all incidents and the corrective measures implemented, thereby promoting transparency and accountability in the documentation process.
To illustrate the effectiveness of these regulations, consider that the FDA receives over 2 million reports concerning health equipment each year, highlighting the extent of oversight necessary for equipment safety. Successful manufacturers have demonstrated their ability to consistently meet these documentation deadlines, ensuring that serious incidents are recorded and addressed promptly. For instance, manufacturers must submit reports within 30 days after becoming aware of a reportable event, reinforcing the urgency of compliance.
Recent modifications in healthcare equipment documentation requirements have further enhanced these procedures, emphasizing the necessity for thorough data gathering. Manufacturers are now required to provide a statement explaining any missing information and the steps taken to obtain it, thereby improving the quality of reports submitted to the FDA. This advancement in documentation methods reflects a commitment to enhancing patient well-being and equipment effectiveness.
Overall, the average time taken for manufacturers to report serious incidents is pivotal in evaluating the effectiveness of the framework related to the MDR full form in medical. By adhering to these stringent timelines for submission and maintaining detailed records, manufacturers significantly contribute to the ongoing effort to ensure the security and effectiveness of healthcare products in the marketplace.
Voluntary Medical Device Reporting: Expanding Compliance Beyond Requirements
Voluntary medical device notifications empower manufacturers to disclose incidents that may not meet the criteria for mandatory reporting. This proactive strategy is crucial for the early identification of potential issues, enabling timely corrective actions that can mitigate risks. Organizations that embrace voluntary disclosures enhance their commitment to patient safety while strengthening their regulatory compliance.
Research indicates that constructive feedback regarding errors significantly increases the likelihood of incident documentation, with a pseudo R within-groups for the overall model assessing event frequency at 0.47. Such dedication can markedly improve their market position, showcasing a culture that prioritizes safety and transparency.
As illustrated by PepsiCo's 'Courage to Care' initiative, fostering an environment that encourages reporting can lead to improved outcomes for patient safety. By cultivating a supportive rather than punitive atmosphere, companies can enhance overall patient safety results and contribute to a more resilient healthcare equipment ecosystem.
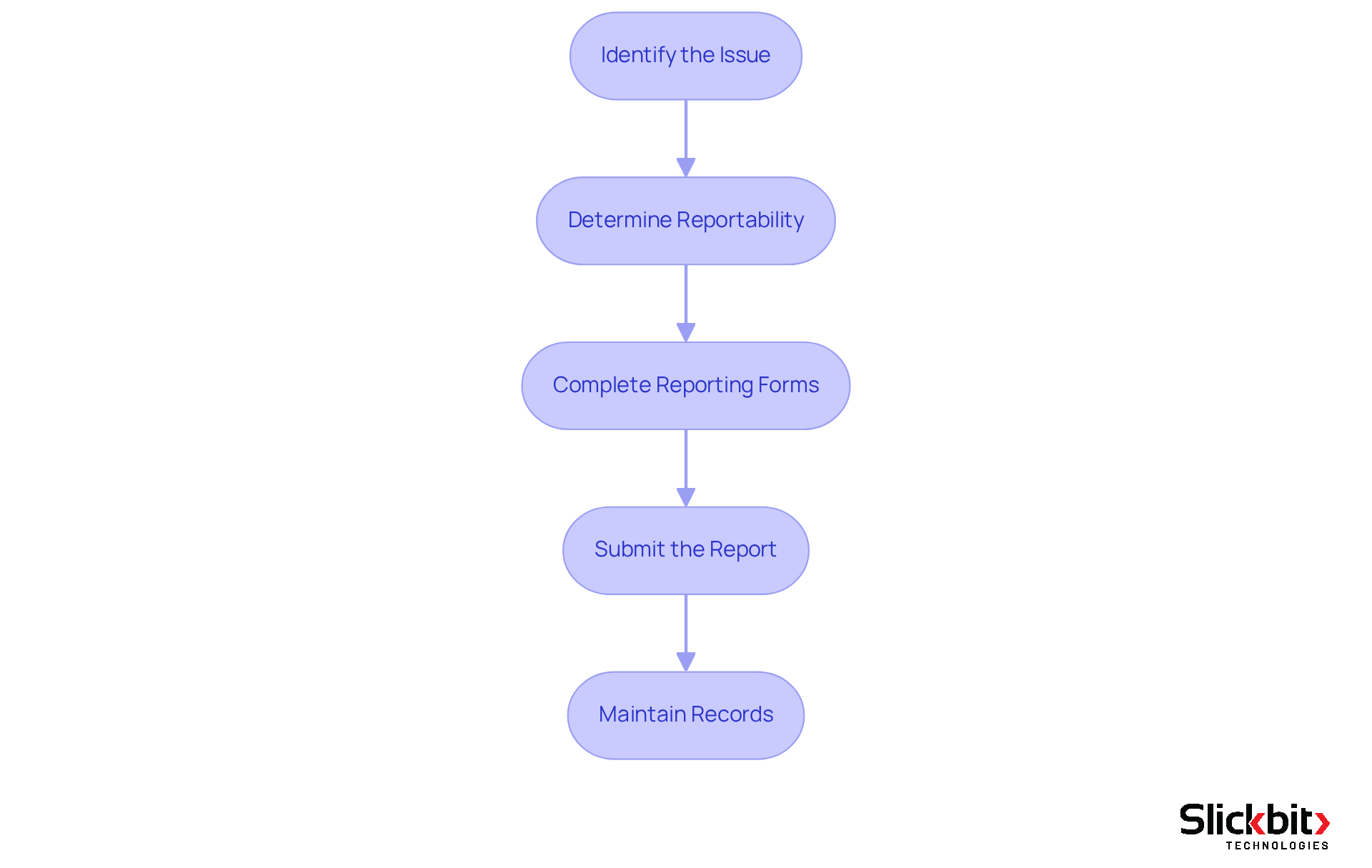
How to Report Medical Device Problems: A Step-by-Step Guide
Effectively reporting medical equipment issues necessitates a systematic approach. To ensure compliance and thorough documentation, follow these essential steps:
- Identify the Issue: Start by clearly defining the problem. Collect all pertinent information, including device specifics, incident descriptions, and any potential patient impact.
- Determine Reportability: Evaluate whether the incident falls under mandatory or voluntary disclosure requirements. Grasping the criteria for reportable events is critical.
- Complete Reporting Forms: Accurately fill out the necessary forms, ensuring all required information is included. This may involve using specific forms such as the FDA's Form 3500A for mandatory reports.
- Submit the Report: Dispatch the completed report to the appropriate regulatory authority within the designated timeframe. For instance, the FDA mandates that adverse events be communicated within 30 days of awareness, underscoring the importance of prompt notification.
- Maintain Records: Keep meticulous records of the report and any follow-up actions taken. This documentation is vital for compliance and future reference.
Regulatory specialists emphasize that prompt and precise documentation is not merely a regulatory obligation but a commitment to patient well-being. As Mike Drues, president of Vascular Sciences, aptly notes, "no one is going to get into trouble for over-reporting, but for under-reporting." This statement highlights the ethical responsibility manufacturers bear in effectively communicating all potential safety issues. Moreover, it is noteworthy that over 1.2 million medical device adverse event submissions were not provided to the FDA within the legal timeframe, illustrating the serious repercussions of delayed notifications.
Successful reporting processes frequently involve proactive engagement with regulatory bodies, which can offer guidance on submission strategies and streamline the reporting process. Additionally, implementing a robust document and quality management system (QMS) is advisable to ensure compliance with regulatory standards and uphold data integrity. By prioritizing clear communication and comprehensive documentation, organizations can enhance their compliance initiatives and contribute to improved patient welfare outcomes.
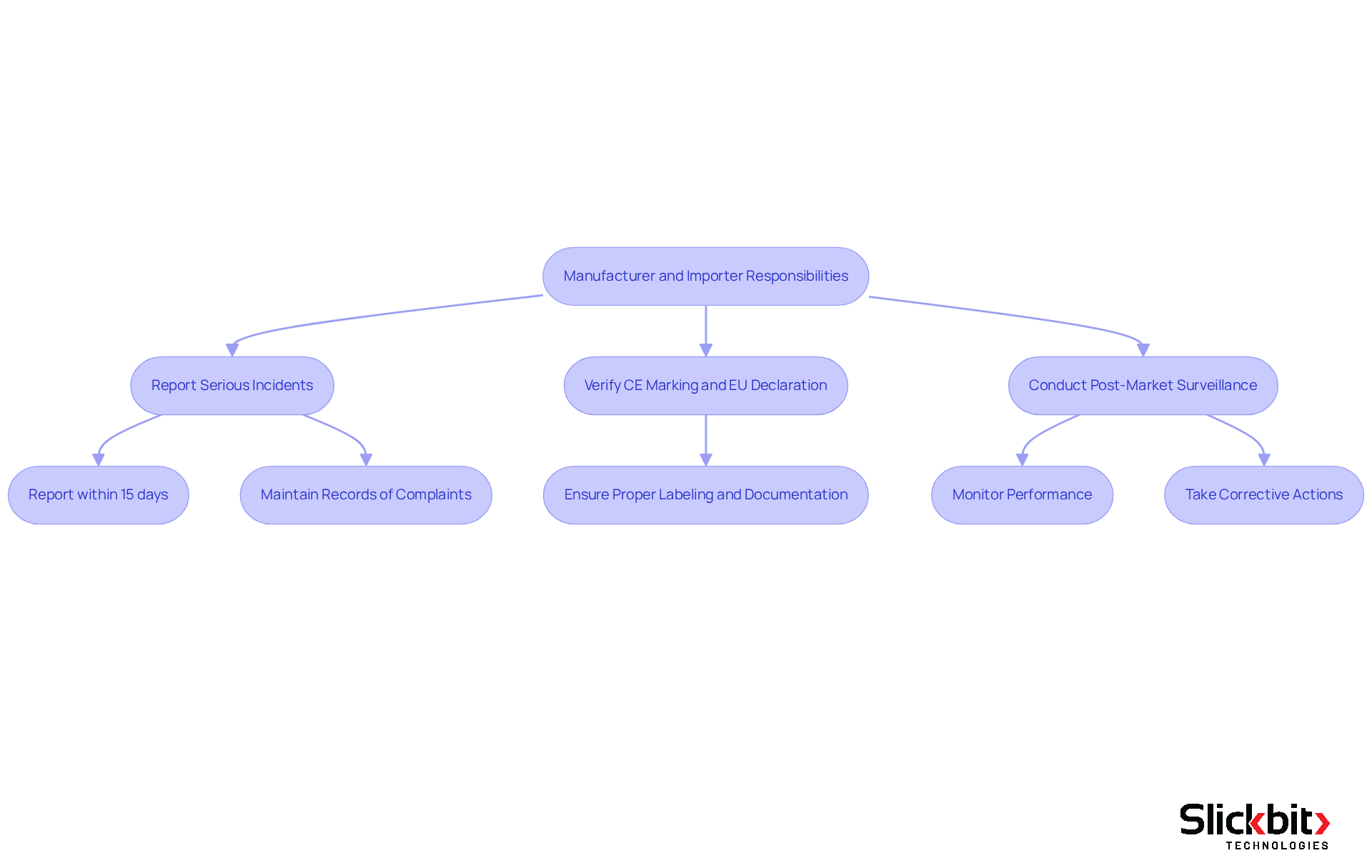
Manufacturer and Importer Responsibilities in Medical Device Reporting
Under the Medical Device Regulation (MDR), known as the mdr full form in medical, producers and importers bear essential responsibilities to ensure the safety and efficacy of medical devices. They are required to report any serious incidents promptly and to maintain comprehensive records of all complaints and adverse events. The reporting rates of adverse events are crucial for regulatory compliance and patient safety; for example, manufacturers must report serious incidents within 15 days, emphasizing the urgency of their obligations.
Importers, in particular, have specific responsibilities, including verifying that products are CE marked and possess an EU declaration of conformity before they can be marketed. Non-compliance with these verification procedures can result in significant repercussions, including a ban on the sale of equipment until compliance is achieved. A case study on the General Obligations of Importers illustrates this necessity, underscoring the importance of proper labeling and documentation to avoid non-compliance.
Post-market surveillance represents another critical aspect of their responsibilities. Producers are mandated to actively monitor the performance of their products once they have entered the market. Companies have effectively implemented robust post-market surveillance strategies, enabling them to identify potential issues early and take corrective actions as needed. This proactive approach not only ensures compliance but also enhances the overall safety profile of their products.
Additionally, resources such as the UDI EUDAMED tool and complimentary MDR Gap Analysis services are available to support manufacturers and importers in fulfilling their regulatory obligations effectively.
In conclusion, the duties outlined in the MDR full form in medical are designed to foster a culture of protection and accountability within the healthcare equipment industry, ensuring that both producers and importers play a vital role in safeguarding public health.
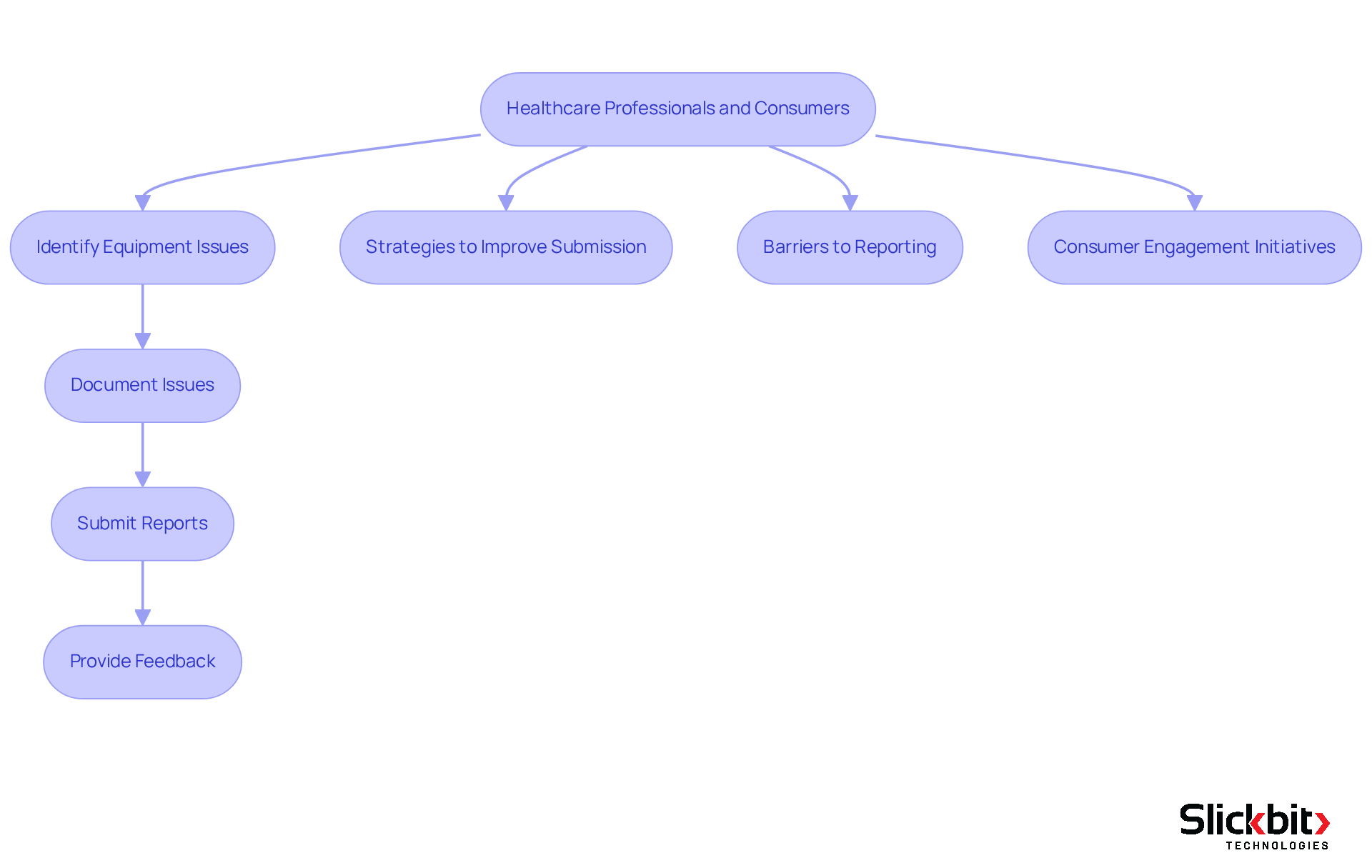
The Role of Healthcare Professionals and Consumers in Reporting
Healthcare specialists and users play a vital role in the documentation of health equipment, often serving as the first line of defense in identifying equipment-related issues. Their insights are invaluable, providing manufacturers and regulatory bodies with critical information that can expedite the recognition of concerns and enhance response strategies. For instance, an examination by the UK National Patient Security Agency revealed that 1,021 out of 12,084 patient incident occurrences were linked to medical devices, underscoring the necessity for robust documentation systems.
Encouraging open communication among these stakeholders cultivates a culture of safety and vigilance. Strategies to improve consumer submission rates include:
- Implementing user-friendly electronic submission systems
- Providing comprehensive training on submission procedures
Research indicates that fear of repercussions and uncertainty regarding protocols for reporting incidents are significant barriers to effective documentation, which can be mitigated through education and support. As noted by the authors, "Strategies to enhance error documentation involve the use of an electronic error documentation system, increased training and feedback to frontline clinicians about the reported mistake."
Instances of consumer engagement in healthcare product safety documentation are increasingly evident. Initiatives such as patient feedback systems and community outreach programs empower patients to report issues directly, resulting in more timely interventions. For example, digital platforms that allow patients to share their experiences with health products can strengthen the feedback loop between consumers and producers. As healthcare evolves, the integration of telehealth and digital tools will further amplify consumer participation in issue communication, ensuring that patient experiences and concerns are recognized and addressed. The FDA defines a healthcare instrument as a tool used to identify, treat, or prevent illnesses without chemical effects in the body, highlighting the importance of accurate documentation in ensuring equipment safety.
Ultimately, the collaboration between healthcare professionals and patients is essential for enhancing the safety of health equipment. By actively participating in documentation, consumers contribute to creating a safer healthcare environment, fostering advancements in equipment design and regulatory oversight.
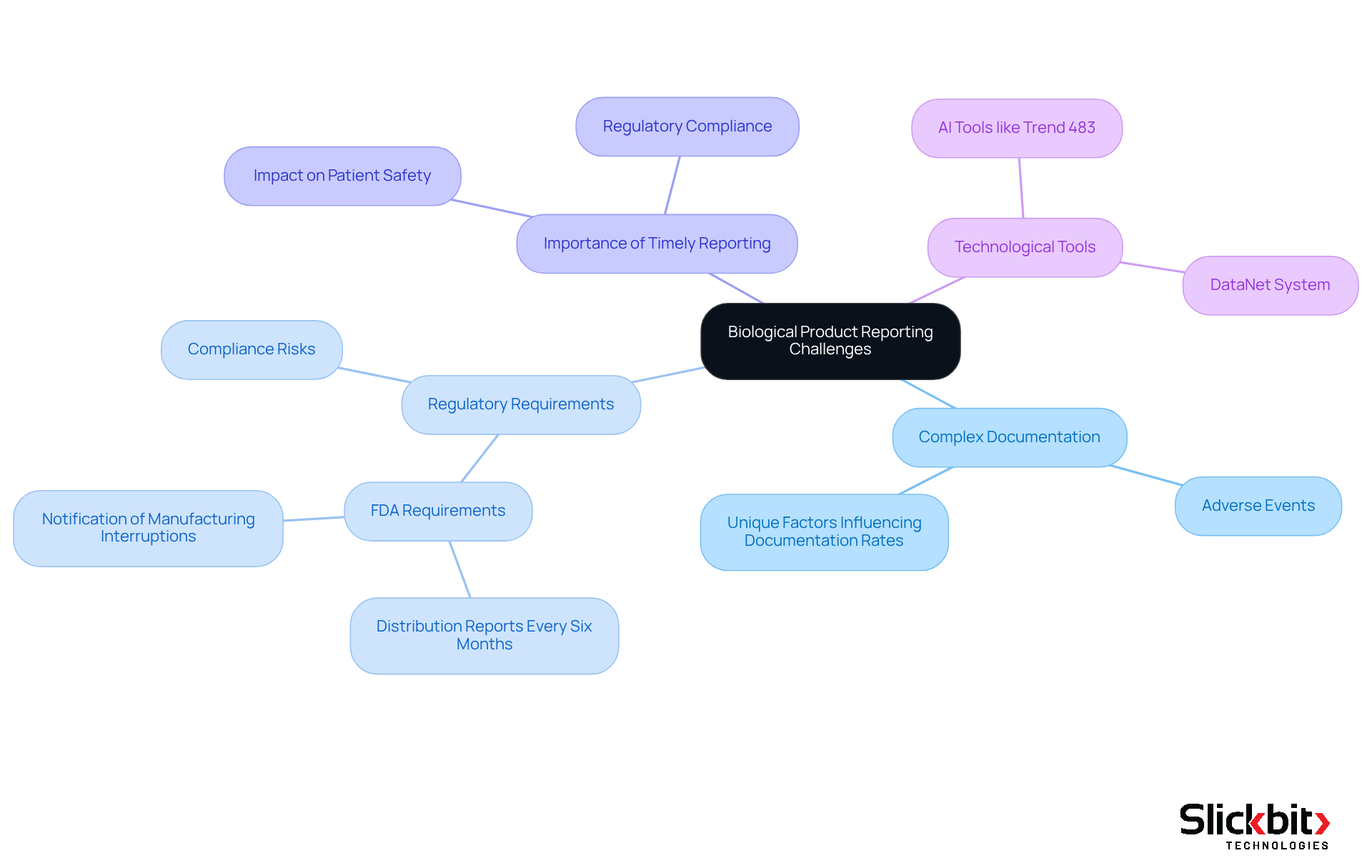
Submitting Reports for Biological Products: Unique Considerations
Reporting for biological products presents distinct challenges due to their inherent complexity. Manufacturers must navigate specific documentation requirements that differ from those applicable to conventional medical devices. Key considerations include understanding the implications of adverse events, which can significantly impact patient safety and regulatory compliance. For instance, the FDA mandates that manufacturers notify them in writing of any permanent discontinuance or interruption in manufacturing that could disrupt supply, particularly for life-supporting products. This requirement highlights the significance of timely and accurate documentation.
Moreover, the adverse event documentation rates for biological products can vary, necessitating a thorough understanding of the unique factors influencing these rates. Manufacturers must ensure that all relevant data is meticulously documented and submitted to the appropriate authorities, as failure to do so can lead to regulatory repercussions and jeopardize patient safety. The complexities of biological product documentation are further illustrated by the FDA's requirement for distribution records every six months, detailing the quantity of biologics distributed, including specific lot numbers and expiration dates. This level of scrutiny emphasizes the essential nature of precise documentation in sustaining supply chain integrity and public health.
In this context, tools like Slickbit's Trend 483 can be invaluable. By utilizing AI to detect trends in adverse event documentation and compliance, manufacturers can obtain deeper insights into their processes. This technology can streamline the documentation and submission of reports, ensuring that all relevant data is captured efficiently and accurately.
As Francisco Laranjeira, a Superior Technician for Health and Geneticist, noted, "To accurately and securely monitor storage conditions of the biological samples and critical products used in laboratory analysis was already a challenge and a delicate procedure." This viewpoint highlights the real-world complexities encountered by manufacturers in ensuring adherence to strict documentation requirements.
In summary, the landscape of biological product documentation is characterized by its unique challenges and stringent requirements, necessitating a proactive approach from manufacturers to ensure compliance and safeguard patient welfare. The implementation of systems like DataNet, alongside AI tools such as Trend 483, can aid in meeting these health regulations, ensuring that manufacturers can effectively navigate the complexities of reporting.
Searching Medical Device Reports: Tools and Techniques
To effectively search healthcare equipment reports, it is essential to utilize online databases such as the FDA's MAUDE database and the EUDAMED platform. Begin by employing advanced search methods, including:
- Keyword queries
- Specific filters for types of equipment
- Designated date ranges to refine your results
Familiarizing yourself with the structure and functionalities of these databases can significantly enhance your capability to uncover relevant reports and insights. Consequently, this knowledge empowers you to navigate the complexities of healthcare equipment data with confidence.
Additional Resources for Medical Device Reporting: Where to Find Help
Organizations seeking assistance in health equipment documentation have access to a wealth of resources, including regulatory authority websites, industry associations, and specialized training programs. Key regulatory organizations, such as the FDA and EMA, provide comprehensive guidelines and updates on documentation requirements, ensuring adherence to the latest standards. Furthermore, industry associations often conduct workshops and seminars that emphasize best practices in medical device documentation, with participation rates frequently surpassing 70%. This statistic underscores the significance of these events in the field.
The integration of AI technologies, exemplified by those used in Trend 483, enhances the understanding of systemic risks and compliance trends. These AI systems empower users to search, filter, and view complete FDA 483s directly, offering deeper insights into inspections that can guide documentation practices. Additionally, training programs have been shown to notably enhance compliance rates, with some organizations reporting a 40% increase in adherence to standards after participation. Consequently, for comprehensive guidelines on the mdr full form in medical device reporting, organizations can refer to resources available on the websites of these regulatory authorities and industry associations, which provide invaluable tools for navigating the complexities of compliance.
To maximize the benefits, consider enrolling in relevant training programs and actively participating in industry workshops.
Conclusion
The exploration of the MDR full form in medical device reporting underscores its critical role in ensuring safety and compliance within the healthcare industry. By delving into the nuances of medical device definitions, mandatory reporting requirements, and the responsibilities of manufacturers and importers, stakeholders can effectively navigate the complex landscape of medical device regulation. Furthermore, the integration of AI technologies, such as those offered by Slickbit, significantly enhances compliance efforts, streamlining documentation processes and improving patient outcomes.
Key insights highlighted throughout this article emphasize the importance of not only adhering to mandatory reporting but also embracing voluntary reporting practices. This proactive approach fosters a culture of safety and accountability, enabling manufacturers to identify potential issues early and mitigate risks effectively. Moreover, collaboration among healthcare professionals, consumers, and regulatory bodies is essential for enhancing the overall safety of medical devices.
Ultimately, the significance of robust medical device reporting cannot be overstated. As the healthcare sector continues to evolve, stakeholders are encouraged to prioritize compliance and actively engage in reporting practices. By leveraging available resources, participating in training programs, and utilizing advanced tools for documentation, organizations can ensure they meet regulatory standards while contributing to a safer healthcare environment.
Frequently Asked Questions
What is Slickbit and what services does it provide?
Slickbit is a company that delivers advanced AI solutions to enhance adherence in healthcare equipment documentation, automating processes to ensure accuracy and compliance with regulatory requirements.
How does Slickbit improve medical device reporting compliance?
Slickbit leverages AI-driven analytics to automate documentation processes, streamline workflows, and reduce the risk of errors, which is crucial for maintaining regulatory standards.
What is the significance of AI in healthcare according to the article?
The article highlights that 96% of surveyed leaders believe AI can enhance patient outcomes and experiences, emphasizing its transformative potential in improving compliance within the healthcare sector.
What is Lumino, and how does it assist pharma teams?
Lumino is Slickbit's AI-powered Regulatory Intelligence assistant that provides pharma teams with accurate, traceable answers from FDA and global guidance documents.
What role does Vault Redact play in medical documentation?
Vault Redact automates the identification and removal of Personally Identifiable Information (PII) and Protected Health Information (PHI) from documents, ensuring secure content management.
What is the definition of a medical device under the MDR?
A medical device is defined as any instrument, apparatus, appliance, software, implant, reagent, or material intended for medical purposes, ranging from simple bandages to complex diagnostic software.
Why is understanding the definition of medical devices important for producers and importers?
It helps them identify which products fall under the MDR's jurisdiction and require appropriate documentation, which is essential for compliance and operational success.
What are the mandatory reporting requirements for manufacturers under the MDR?
Manufacturers must report serious incidents and field corrective actions to relevant authorities within 10 days of becoming aware of a serious incident, which includes device-related deaths or serious injuries.
How does the FDA monitor health equipment safety?
The FDA receives over 2 million reports concerning health equipment each year, highlighting the extensive oversight needed to ensure equipment safety.
What recent modifications have been made to healthcare equipment documentation requirements?
Manufacturers are now required to provide a statement explaining any missing information and the steps taken to obtain it, which improves the quality of reports submitted to the FDA.
Why is timely reporting of serious incidents important?
Adhering to stringent timelines for submission and maintaining detailed records contributes significantly to ensuring the security and effectiveness of healthcare products in the marketplace.




