Overview
The primary focus of this article is to deliver critical insights on EU Annex 11 compliance tailored specifically for pharmaceutical R&D managers. Understanding compliance requirements is paramount in today's regulatory environment. Furthermore, the adoption of AI technologies can significantly enhance operational efficiency, enabling managers to navigate complexities with greater ease. In addition, implementing robust documentation and training practices is essential for effectively adapting to the evolving regulatory landscape. By prioritizing these strategies, R&D managers can ensure not only compliance but also drive innovation within their organizations.
Introduction
The landscape of pharmaceutical research and development is experiencing a profound transformation as the European Union's Annex 11 regulations evolve. These changes demand a more rigorous approach to data integrity, security, and compliance, presenting R&D managers with a unique opportunity.
By harnessing advanced AI technologies, organizations can streamline adherence processes and significantly enhance operational efficiency. However, as they strive to meet these new standards, they encounter substantial challenges, including outdated systems and insufficient training.
Consequently, how can R&D leaders effectively navigate this complex regulatory environment while ensuring their teams remain compliant and agile?
Slickbit: AI Solutions for Streamlining EU Annex 11 Compliance
Slickbit.ai presents advanced AI solutions specifically designed to ensure compliance with the regulations of EU Annex 11. By leveraging cutting-edge technologies, including real-time adherence guidance and AI-powered Regulatory Intelligence, Slickbit empowers Life Sciences organizations to automate and enhance their workflow processes. This not only guarantees conformity to regulatory standards but does so with remarkable efficiency. Notably, 44% of regulatory officers are already harnessing AI to boost operational efficiency, underscoring the significant influence of AI on adherence processes.
The rapid evolution of enterprise-level Minimum Viable Products (MVPs) enables R&D managers to swiftly implement these solutions, substantially reducing the time and resources typically required for regulatory efforts. This approach accelerates regulatory processes and allows teams to focus on strategic initiatives, ultimately enhancing productivity and operational excellence within the pharmaceutical sector. Moreover, solutions like Vault Redact automate the identification and removal of PII and PHI from documents, further strengthening adherence and security in sensitive environments.

Understanding EU Annex 11: Key Compliance Requirements for Life Sciences
EU Annex 11 establishes critical criteria for computerized networks within the pharmaceutical sector, highlighting the importance of information integrity, security, and traceability. Regulatory requirements necessitate the verification of frameworks, secure storage and retrieval of data, and the maintenance of comprehensive audit trails. It is imperative for R&D managers to fully comprehend these requirements to ensure compliance and minimize the risk of regulatory penalties.
The 2025 update of EU Annex 11 marks a significant shift towards digital transformation in the pharmaceutical industry, introducing new expectations for data management and process validation. Organizations are urged to adopt a proactive, risk-based approach, investing in staff training and governance to adeptly navigate the complexities of regulations.
Real-world examples underscore the challenges encountered by Life Sciences organizations. Notably, an estimated 60-80% of existing computerized systems may necessitate modifications to align with the updated standards, particularly concerning legacy systems and third-party software that lack essential audit functionalities. Tools such as Slickbit's Trend 483, Lumino, and Vault Redact can facilitate the automation of compliance gap identification and enhance operational efficiency.
Experts stress the critical nature of data integrity and security in this context. As highlighted, "the scale and complexity of the proposed Section 11 requirements demand a collaborative approach between industry and regulators to ensure successful implementation without compromising pharmaceutical innovation or supply chain stability."
In conclusion, understanding the key aspects of EU Annex 11 is essential for Life Sciences organizations aiming to enhance compliance and operational excellence while capitalizing on the opportunities presented by digital advancements. R&D leaders should leverage AI tools like Trend 483, Lumino, and Vault Redact to adeptly navigate the evolving regulatory landscape and ensure their frameworks align with regulatory standards.
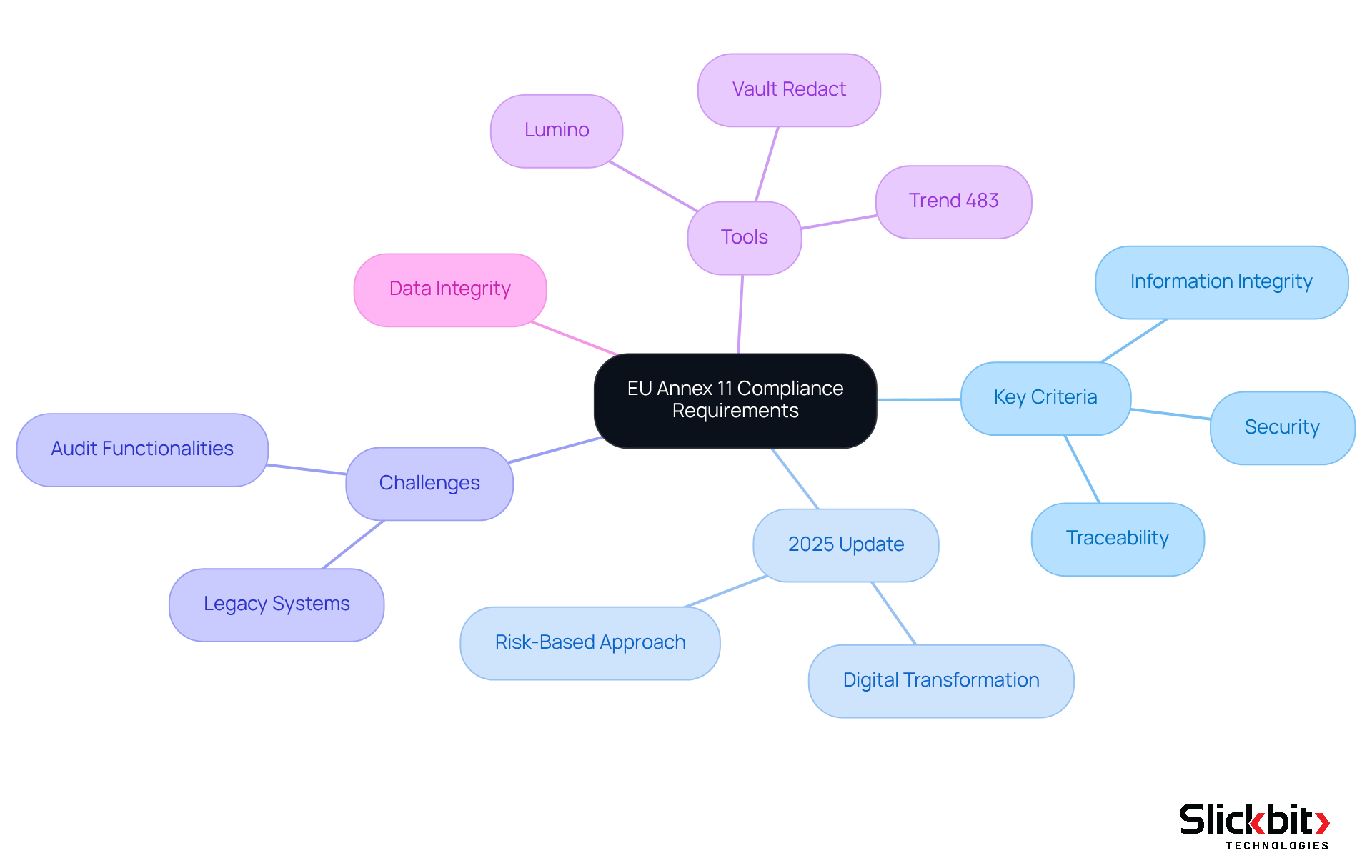
Computerized Systems: Essential Components for EU Annex 11 Compliance
To comply with EU Annex 11, computerized frameworks must incorporate robust hardware, software, and network infrastructure that collectively ensure information integrity and security. Key components include systems capable of generating accurate and reliable information, maintaining comprehensive audit trails, and enforcing stringent user access controls. R&D managers should focus on selecting and implementing solutions that not only fulfill regulatory requirements but also enhance operational efficiency.
Current trends indicate a shift towards integrated electronic Quality Management Systems (eQMS) that streamline regulatory processes while bolstering information governance. Furthermore, the adoption of advanced security measures, such as multi-factor authentication and encryption, is becoming standard practice to protect sensitive information.
Best practices dictate that organizations perform thorough risk assessments to identify critical systems and apply appropriate validation and documentation controls. This proactive approach not only mitigates regulatory risks but also fosters a culture of continuous improvement in data management practices. Moreover, Slickbit's Vault Redact solution automates the identification and removal of PII and PHI from documents, further supporting compliance initiatives. Adherence to EU Annex 11 is recognized as a best practice within the pharmaceutical and life sciences sectors, emphasizing its importance in ensuring product quality and process control. By prioritizing these elements, pharmaceutical firms can guarantee that their computerized infrastructures are not only compliant but also resilient and effective in supporting their operational objectives.
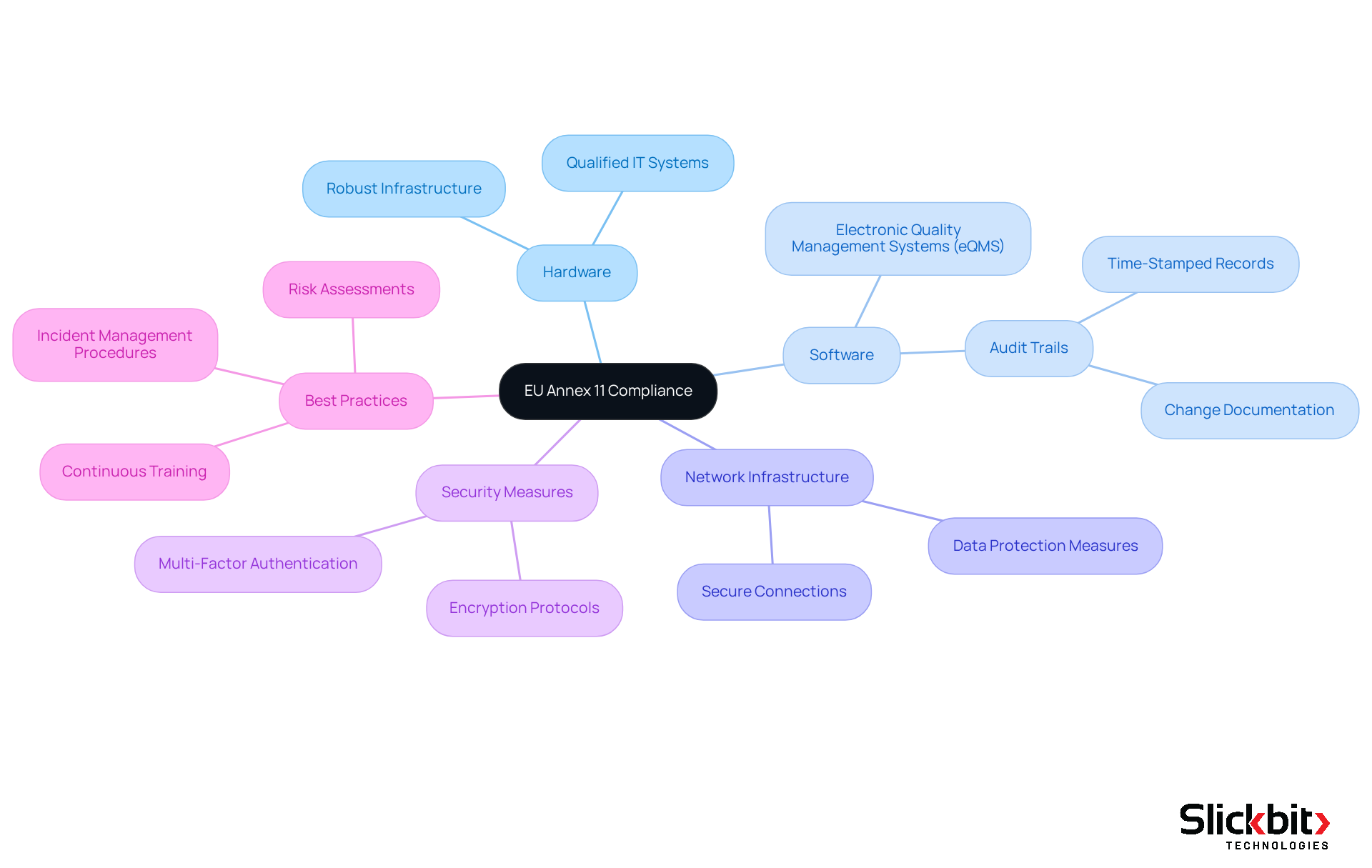
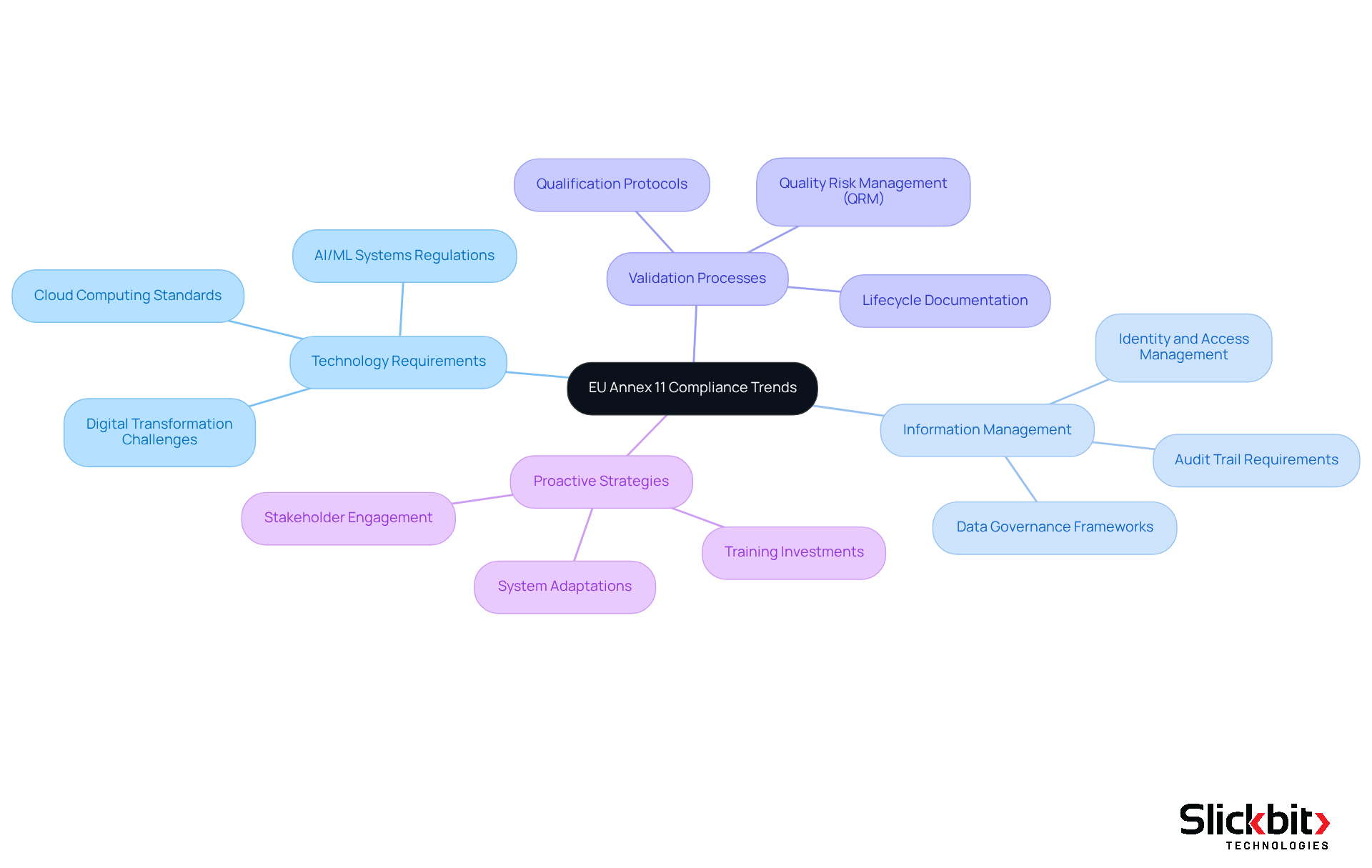
Validation Processes: Ensuring Compliance with EU Annex 11 Standards
To guarantee adherence to EU Annex 11 standards, it is imperative that validation procedures for computerized setups are robust and thoroughly documented. This necessitates the creation of clear validation protocols, comprehensive risk assessments, and regular checks of the network. R&D managers must implement a structured validation framework that complies with EU Annex 11 and encompasses all phases of development and deployment, ensuring compliance from the outset.
A proactive, risk-based approach is essential; it enables organizations to identify and effectively mitigate potential risks associated with computerized systems. Compliance specialists emphasize that embedding quality into digital operations requires robust integrity controls throughout the lifecycle of information, ensuring that it remains attributable, legible, contemporaneous, original, and accurate (ALCOA).
Furthermore, by utilizing AI tools such as Trend 483, R&D managers can gain valuable insights into regulatory trends and systemic risks, enhancing their capability to uphold data integrity and ensure that all electronic records and signatures comply with the required standards for authenticity and traceability.
Ongoing system validation and formal change control procedures are critical for adapting to evolving regulatory requirements. This thorough methodology not only safeguards against regulatory violations but also empowers organizations to respond swiftly to audits and security concerns.
As Dipti Dhakul states, 'In banking, adherence to regulations is not optional; it’s the foundation.' R&D managers should adopt these practices to enhance their adherence stance and operational efficiency.
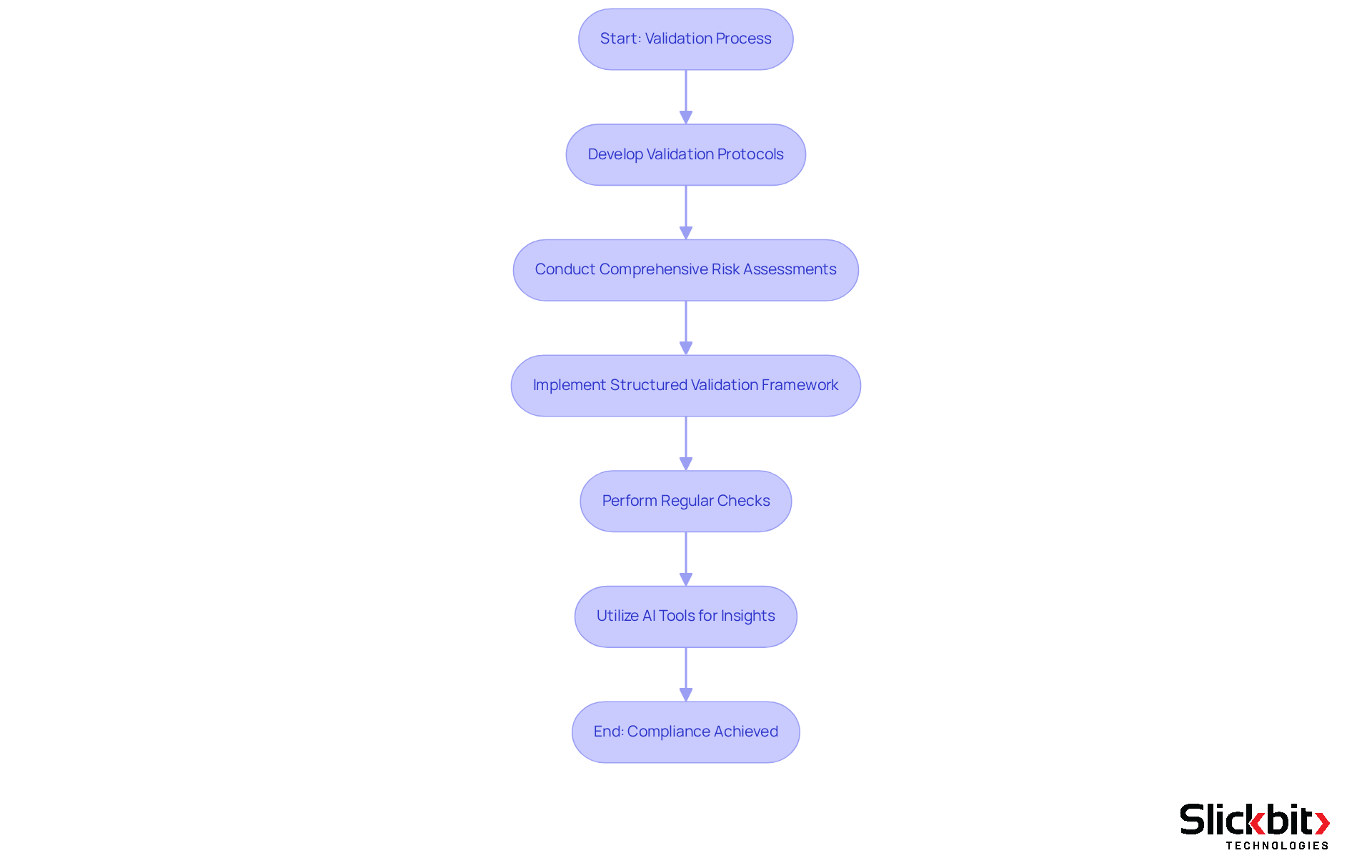
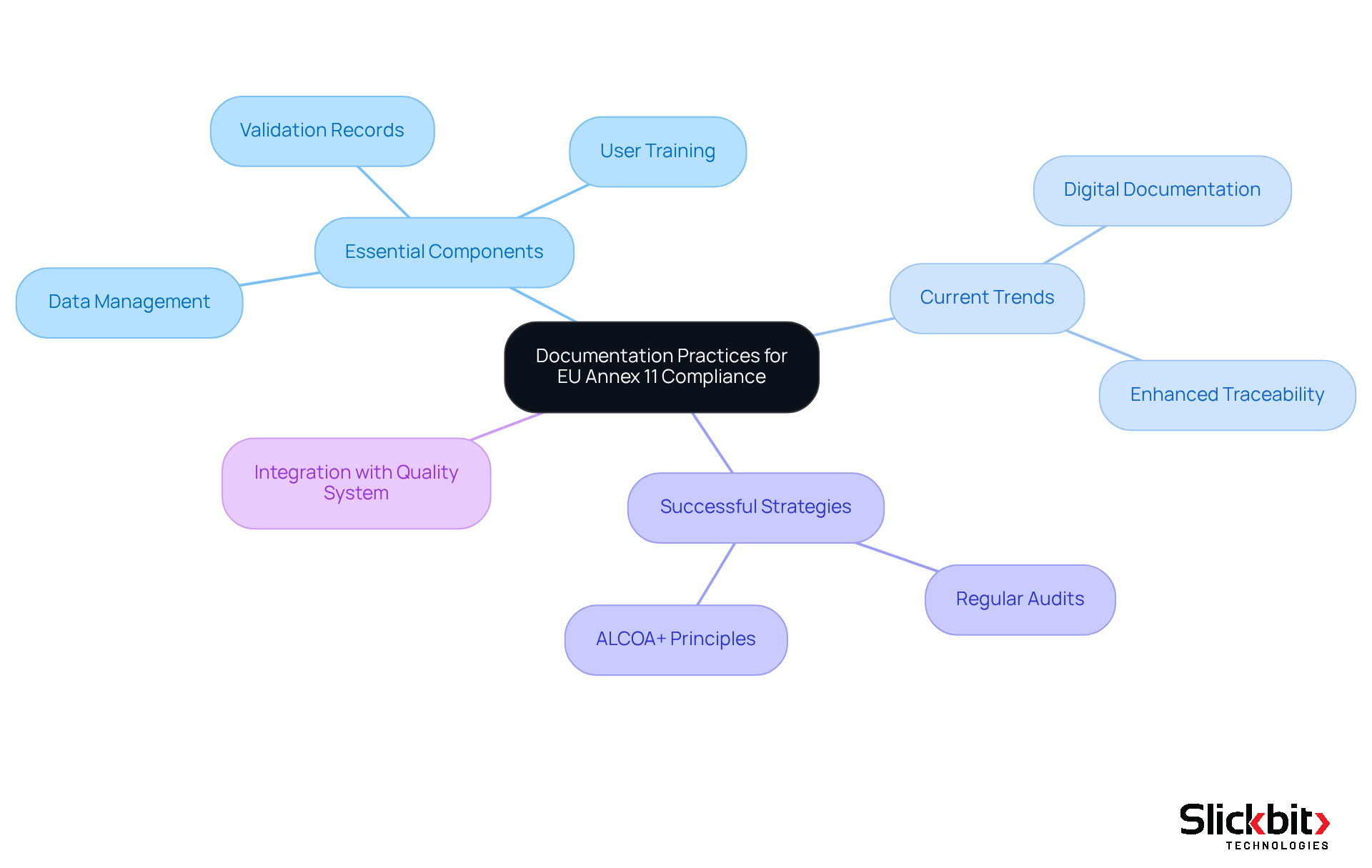
Documentation Practices: Supporting EU Annex 11 Compliance Efforts
Effective documentation practices are essential for achieving compliance with EU Annex 11. This requirement involves maintaining comprehensive records of validation, user training, and data management processes. R&D managers must implement robust documentation protocols that ensure all compliance-related activities are accurately recorded and readily accessible for audits and inspections.
Current trends reveal a shift towards digital documentation methods that enhance traceability and streamline access to critical information. Successful documentation strategies encompass:
- Regular audits of records
- Ensuring alignment with ALCOA+ principles—Attributable, Legible, Contemporaneous, Original, Accurate, and complete.
Insights from regulatory professionals underscore the necessity of comprehensive records to demonstrate adherence and facilitate smoother audits. To effectively maintain records for EU Annex 11 audits, organizations should prioritize integrating their documentation practices with their Pharmaceutical Quality System. This integration ensures that all computerized systems are evaluated for GMP applicability and that mandatory audit trails are established. Such a proactive approach not only helps ensure adherence but also enhances overall operational integrity.
Training and Awareness: Building a Compliance Culture for EU Annex 11
Creating a robust regulatory culture within life sciences organizations is essential, hinging on continuous training and awareness programs tailored for all staff engaged in R&D processes. R&D managers must prioritize regular training sessions that delve into the requirements of EU Annex 11, underscoring the critical importance of data integrity and the role of computerized systems in maintaining standards.
Furthermore, integrating AI tools such as Slickbit's Trend 483 can significantly enhance these training initiatives by providing insights into systemic risks and regulatory trends identified through FDA inspections. This proactive strategy not only clarifies individual responsibilities but also reinforces the overarching significance of compliance in everyday operations.
By fostering an environment where adherence is viewed as vital to success, organizations can bolster their operational integrity and mitigate risks associated with regulatory violations. In addition, implementing feedback mechanisms within training programs can greatly improve the comprehension and retention of regulatory obligations, ensuring that teams remain informed and prepared to navigate the complexities of legal requirements.
Integrating AI Technologies: Enhancing Compliance with EU Annex 11
Incorporating AI technologies into regulatory processes offers substantial advantages for meeting the standards of EU Annex 11. By automating data management, AI enhances precision in reporting and facilitates real-time oversight of regulatory activities. For instance, AI can significantly reduce the time spent on manual obligation extraction from regulatory texts, which typically exceeds five hours per obligation, to mere minutes. This transformation not only decreases the potential for human error—previously noted to reach a 14.6% error rate in manual processes—but also streamlines overall efficiency.
R&D managers should consider AI solutions such as:
- Slickbit's Vault Redact, which simplifies the detection and removal of PII and PHI from documents.
- Trend 483, which utilizes AI to identify patterns in systemic risks and compliance from FDA 483s.
- The AI-powered Regulatory Intelligence assistant, Lumino, which enables pharma teams to access accurate, traceable answers from FDA and global guidance documents.
These tools integrate seamlessly with existing systems like Veeva or Medidata, creating a comprehensive regulatory framework. They not only simplify reporting but also provide immediate notifications and actionable insights, promoting proactive decision-making and continuous monitoring of regulatory metrics. With regulatory costs expected to rise by 6-9% annually through 2030, leveraging AI can mitigate these expenses by reducing manual effort and enhancing operational efficiency.
Current trends indicate a shift towards real-time adherence monitoring, crucial in a landscape where traditional periodic checks are increasingly inadequate. Organizations that rely on manual processes face 3.2 times more violations than those employing automation. AI-driven solutions empower regulatory teams by filtering out up to 95% of irrelevant alerts, allowing them to focus on critical regulatory issues. This proactive strategy not only aids organizations in avoiding significant penalties linked to regulatory breaches but also positions them to adapt swiftly to evolving regulations. As emphasized by industry specialists, the integration of AI in regulatory processes transcends mere technological enhancement; it is a strategic imperative for maintaining regulatory compliance and ensuring patient safety within the pharmaceutical sector.
Challenges in Achieving EU Annex 11 Compliance: Common Pitfalls and Solutions
Achieving conformity with EU Annex 11 poses significant challenges for R&D managers, primarily due to common pitfalls such as inadequate documentation, insufficient training, and ineffective validation processes. Experts emphasize that many organizations struggle with absent or partial records, which can lead to regulatory breaches and unsuccessful audits. To combat these issues, R&D managers must implement clear documentation protocols that incorporate standardized templates and regular review reminders for Standard Operating Procedures (SOPs).
Investing in comprehensive training programs is essential, as numerous employees lack a thorough understanding of documentation standards, resulting in inconsistent record-keeping. Regular training refreshers and practical case studies can enhance staff skills and ensure compliance with regulatory requirements. Furthermore, establishing robust validation frameworks, such as automated auditing tools, simplifies regulatory efforts by providing real-time notifications for documentation errors and ensuring that all records remain current and accessible.
By proactively addressing these prevalent challenges, R&D managers can significantly improve their organizations' compliance with EU Annex 11 regulations, ultimately enhancing operational efficiency and reducing the risk of regulatory penalties.
Continuous Monitoring: Ensuring Ongoing Compliance with EU Annex 11
Ongoing assessment of adherence activities is vital for maintaining compliance with EU Annex 11 standards. R&D managers must implement systems that facilitate real-time monitoring of adherence metrics, enabling the rapid identification of potential issues. This proactive strategy not only bolsters risk management but also ensures organizations can swiftly adapt to regulatory changes.
Recent advancements in oversight monitoring technologies, particularly AI-based solutions like Slickbit.ai's Trend 483 tool, streamline the regulatory process through automated information gathering and analysis. For example, AI algorithms can scrutinize historical data from FDA 483s to uncover systemic risks and recurring violations, allowing for the anticipation of new regulations' impacts.
Furthermore, automated auditing tools can perform real-time checks and flag potential issues for review, significantly alleviating the burden on regulatory teams. Regular audits and evaluations of adherence processes are crucial for effectively addressing any regulatory changes, including those related to EU Annex 11, ultimately cultivating a culture of compliance that enhances overall operational efficiency.
Future Trends in EU Annex 11 Compliance: Preparing for Regulatory Changes
As the regulatory environment evolves, R&D managers must remain vigilant to emerging trends in EU Annex 11 regulations. Anticipating shifts in technology requirements, information management practices, and validation processes is essential for compliance.
Industry leaders assert that proactive preparation is vital for maintaining robust compliance efforts. Organizations are increasingly focused on enhancing their data governance frameworks and updating their validation protocols to align with the new standards. This forward-thinking approach not only mitigates the risk of non-compliance but also positions companies to effectively leverage advancements in technology.
By investing in training and adapting their systems, organizations can adeptly navigate the complexities of the updated regulations, ensuring they meet the stringent requirements established in the EU Annex 11.
Conclusion
Understanding and adhering to EU Annex 11 regulations is paramount for R&D managers in the pharmaceutical sector. Leveraging advanced AI technologies can significantly streamline compliance efforts, ensuring that organizations not only meet regulatory requirements but also enhance operational efficiency. By integrating tools such as Slickbit's AI solutions, R&D teams can automate processes, improve data integrity, and foster a culture of compliance essential in today’s dynamic regulatory landscape.
Key insights reveal the critical need for robust documentation practices, continuous training, and a proactive approach to validation processes. Organizations face challenges such as outdated systems and insufficient training, which can be effectively addressed through the implementation of comprehensive strategies that incorporate both technology and best practices. This dual focus mitigates regulatory risks and positions organizations to capitalize on digital advancements.
As the regulatory environment continues to evolve, it is crucial for R&D managers to stay ahead of emerging trends and prepare for upcoming changes in EU Annex 11 compliance. Investing in AI technologies and fostering a culture of continuous improvement enables organizations to navigate the complexities of regulatory requirements while ensuring patient safety and product quality. Embracing these insights empowers R&D leaders to drive operational excellence and maintain compliance in an increasingly challenging landscape.
Frequently Asked Questions
What is Slickbit and how does it help with EU Annex 11 compliance?
Slickbit is an AI solutions provider that offers advanced technologies to ensure compliance with EU Annex 11 regulations. It provides real-time adherence guidance and AI-powered Regulatory Intelligence to help Life Sciences organizations automate and enhance their workflow processes, ensuring conformity with regulatory standards efficiently.
What are the key features of Slickbit's solutions?
Key features of Slickbit's solutions include real-time adherence guidance, AI-powered Regulatory Intelligence, automation of compliance gap identification, and tools like Vault Redact that automate the removal of Personally Identifiable Information (PII) and Protected Health Information (PHI) from documents.
What are the main compliance requirements of EU Annex 11?
EU Annex 11 outlines critical criteria for computerized networks in the pharmaceutical sector, emphasizing information integrity, security, and traceability. Requirements include data verification, secure storage and retrieval, and maintaining comprehensive audit trails.
How has the 2025 update of EU Annex 11 changed compliance expectations?
The 2025 update introduces new expectations for data management and process validation, urging organizations to adopt a proactive, risk-based approach and invest in staff training and governance to navigate the complexities of regulations effectively.
What challenges do Life Sciences organizations face regarding EU Annex 11 compliance?
Many organizations face challenges in aligning existing computerized systems with updated standards, particularly legacy systems and third-party software that may lack necessary audit functionalities. It is estimated that 60-80% of these systems may require modifications.
What components are essential for computerized systems to comply with EU Annex 11?
Essential components include robust hardware, software, and network infrastructure that ensure information integrity and security, such as systems that generate accurate information, maintain audit trails, and enforce user access controls.
What best practices should organizations follow for EU Annex 11 compliance?
Organizations should conduct thorough risk assessments, apply appropriate validation and documentation controls, and adopt advanced security measures like multi-factor authentication and encryption. Continuous improvement in data management practices is also crucial.
Why is adherence to EU Annex 11 considered a best practice in the pharmaceutical sector?
Adherence to EU Annex 11 is recognized as a best practice because it ensures product quality and process control, ultimately supporting operational objectives and regulatory compliance within the pharmaceutical and life sciences sectors.




