Overview
The article prominently emphasizes the Drug Information Association's (DIA) pivotal role in ensuring compliance within the medical research and development (R&D) sector of pharmaceuticals. By facilitating knowledge exchange, providing educational resources, and fostering collaboration among industry stakeholders, DIA significantly aids organizations in navigating complex regulatory environments. This support enhances the efficiency of drug development processes, ultimately leading to improved outcomes in the pharmaceutical field. As such, the DIA stands as a crucial ally for organizations striving to meet regulatory standards and advance their R&D efforts.
Introduction
The landscape of pharmaceutical research and development is evolving rapidly, making regulatory compliance a critical focal point for success. As organizations strive to navigate this intricate environment, the Drug Information Association (DIA) emerges as a pivotal player, providing essential insights and resources that empower R&D teams. However, the complexity of regulations, coupled with the integration of advanced technologies like AI, raises a pressing question: how can companies effectively leverage DIA’s expertise to enhance their compliance strategies and drive innovation in drug development? This inquiry sets the stage for a deeper exploration of the intersection between regulatory adherence and technological advancement in the pharmaceutical sector.
Slickbit: AI Solutions for Life Sciences R&D
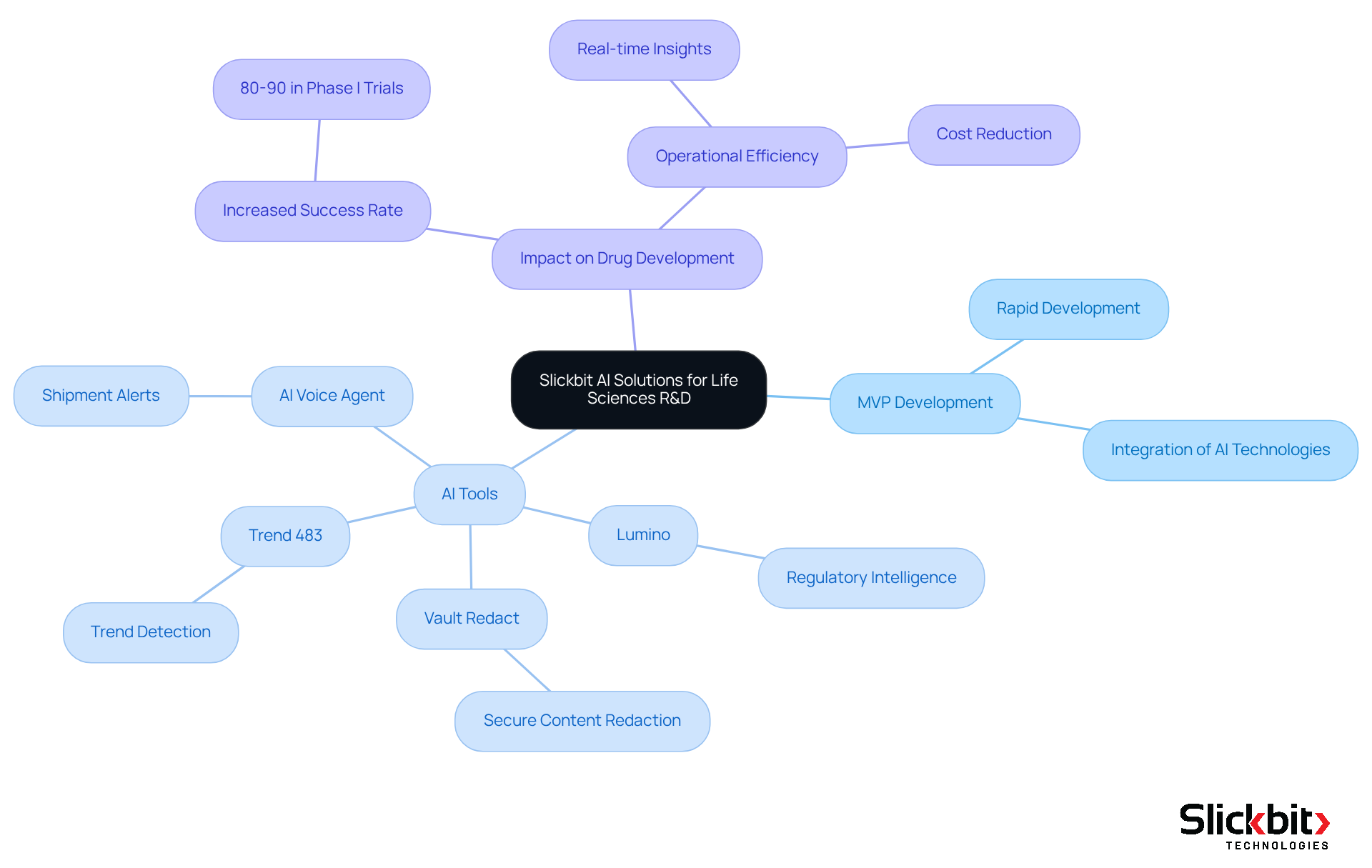
Slickbit.ai stands at the forefront of delivering AI solutions specifically tailored for Life Sciences R&D. The company focuses on rapid MVP development, empowering organizations to seamlessly integrate AI technologies into their drug development processes. By developing AI agents for regulatory and clinical processes—such as Lumino for regulatory intelligence and Vault Redact for secure content redaction—Slickbit ensures that Life Sciences companies can skillfully navigate the complex regulatory environment.
This capability is crucial, as AI-driven drug discovery has demonstrated an impressive 80-90% success rate in Phase I trials, significantly higher than the traditional 40-65% success rate. Furthermore, the incorporation of AI accelerates target identification and enhances adherence through real-time insights, supported by tools like Trend 483 for detecting trends in systemic risks and regulatory concerns.
In addition, Slickbit's AI Voice Agent for Shipment Alerts and AI summarizer further bolster operational efficiency. As the pharmaceutical sector progressively embraces AI, Slickbit's expertise positions it as a vital ally in enhancing R&D regulations and operational efficiency while also facilitating a deeper understanding of the dia full form in medical and DMR within the medical and pharmaceutical frameworks.
Understanding DIA: Importance for Regulatory Compliance
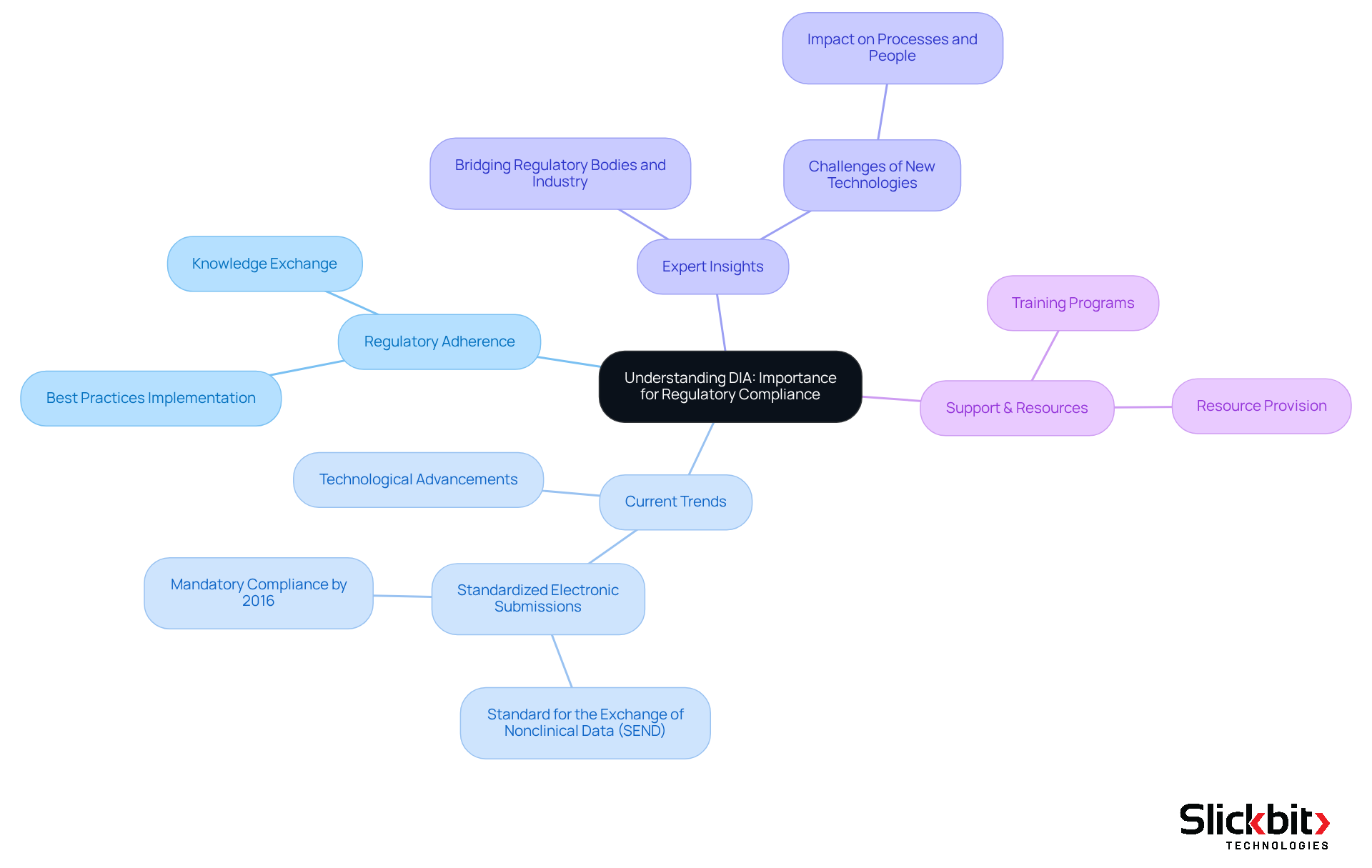
The DIA full form in medical, which stands for Drug Information Association, is pivotal in promoting regulatory adherence within the pharmaceutical sector. By serving as a collaborative platform, the DIA full form in medical facilitates the exchange of knowledge and best practices, empowering organizations to understand and implement the essential regulations governing drug development. This comprehension is crucial for R&D teams, enabling them to navigate the intricate landscape of regulations and safeguard the integrity of their research initiatives.
Current trends reveal an increasing focus on standardized electronic submissions, notably the Standard for the Exchange of Nonclinical Data (SEND), which has emerged as the preferred format for the U.S. FDA. This evolution underscores the importance of organizations like DIA, the DIA full form in medical, in guiding pharmaceutical firms through the shifting compliance landscape.
Expert insights highlight the DIA full form in medical as essential for bridging regulatory bodies and industry participants, ensuring that drug development processes align with regulatory standards. Case studies exemplify how DIA's initiatives have fostered a deeper understanding and application of regulations, ultimately enhancing the efficiency of drug development.
Moreover, DIA actively supports drug development regulations by providing training and resources that help teams adapt to new regulatory challenges. This proactive approach benefits not only the organizations involved but also contributes to the broader advancement of the pharmaceutical industry.
DIA's Role in Enhancing Collaboration in Pharma R&D
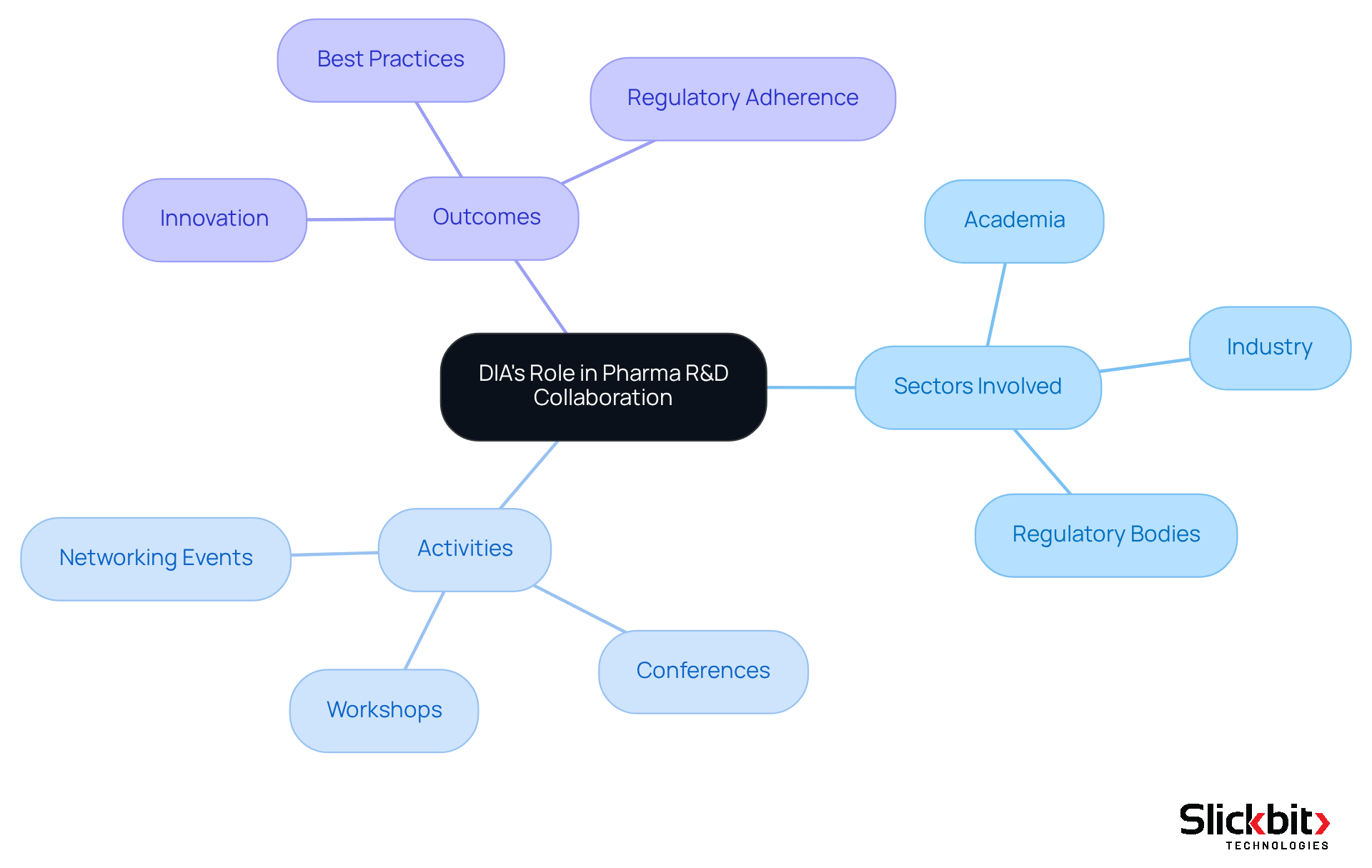
DIA significantly enhances collaboration in pharmaceutical R&D by uniting professionals from diverse sectors, including academia, industry, and regulatory bodies. This initiative is crucial in addressing the complex challenges faced in drug development. Through workshops, conferences, and networking events, DIA fosters an environment that promotes the sharing of ideas and best practices. Such exchanges are essential for encouraging innovation and ensuring adherence to regulatory standards. This cooperative atmosphere empowers R&D groups to share insights and develop solutions that effectively tackle common obstacles in drug creation.
Leveraging DIA Guidelines to Streamline Drug Development
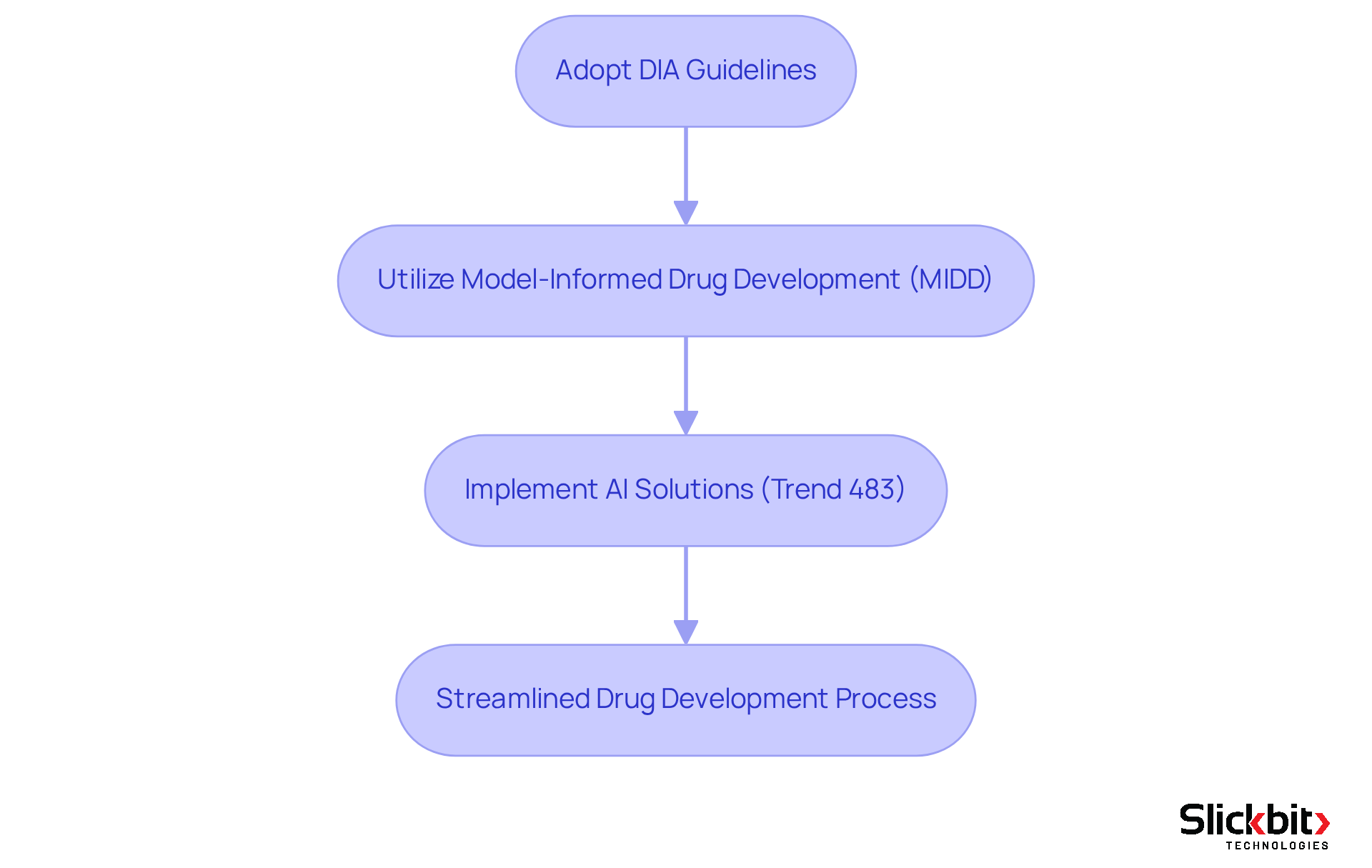
R&D groups can significantly enhance their drug creation processes by employing DIA full form in medical guidelines, which provide a robust framework for best practices in regulatory adherence. Following these guidelines not only reduces delays but also lessens the risk of non-compliance, thereby expediting the time to market for new therapies.
For instance, a study revealed that utilizing Model-Informed Drug Development (MIDD) resulted in annualized average savings of around 10 months of cycle time and $5 million per program.
Furthermore, the integration of AI solutions, such as Slickbit's Trend 483 tool, optimizes this process by identifying trends in systemic risks and compliance issues derived from FDA 483s. Trend 483 allows users to search, filter, and view complete 483s directly for deeper insights, ensuring consistent compliance with standards throughout the process.
Entities that have adopted DIA standards alongside Trend 483 report improved efficiency in managing compliance requirements, which leads to streamlined workflows and reduced timelines. By prioritizing these practices, teams can foster a more agile and compliant approach to drug creation.
DIA's Educational Initiatives for R&D Team Development
DIA offers a comprehensive suite of educational initiatives designed to enhance the expertise of R&D teams within the pharmaceutical sector. These initiatives encompass:
- Workshops
- Webinars
- Certification courses
These programs delve into adherence to regulations, drug development processes, and the latest industry trends. By participating in these programs, R&D professionals can remain informed about best practices and evolving compliance requirements—an essential endeavor given the FDA's issuance of 62 warning letters and 23 import alerts to pharmaceutical companies in FY 2022.
The importance of education in governance adherence is paramount, as it directly influences a company's ability to comply with stringent protocols and mitigate risks associated with violations. For example, the implementation of rigorous quality control measures and regular audits can preempt potential issues, as demonstrated by the over 14,000 drug recalls in the past decade stemming from adverse drug reactions and manufacturing lapses.
DIA's training programs are meticulously crafted to confront these challenges, equipping teams with the essential skills to navigate the complexities of medical R&D. By fostering a culture of continuous learning and regulatory compliance, organizations can enhance their operational efficiency and safeguard their reputations in a highly regulated environment. Moreover, industry-specific training ensures that staff are well-acquainted with the latest advancements, including personalized medicine and digital therapeutics, which are transforming the pharmaceutical landscape.
In conclusion, DIA's educational initiatives are instrumental in empowering R&D teams to adeptly manage compliance, ultimately contributing to the success and integrity of pharmaceutical development.
Networking Opportunities through DIA for R&D Managers
DIA presents a wealth of networking opportunities for R&D managers, empowering them to connect with peers, industry leaders, and compliance specialists. Through conferences, forums, and online communities, R&D professionals can share experiences, discuss challenges, and explore collaborative opportunities. These connections are essential for fostering innovation and ensuring alignment with industry standards and regulatory requirements.
The upcoming DIA 2025 Global Annual Meeting, scheduled for June 15-19 in Washington, will feature over 200 sessions across 12 content tracks, welcoming stakeholders from nearly 50 countries. This event serves as a premier platform for cultivating connections that can lead to successful collaborations, as demonstrated by past initiatives that have emerged from DIA conferences. Notably, six recipients of the Global Inspire Awards and America’s Inspire Awards will be honored during this meeting, highlighting the caliber of participants and the significance of the event.
Networking within the DIA community not only enhances knowledge sharing but also fosters relationships that can accelerate the development of compliant and innovative solutions in the Life Sciences sector. As Emer Cooke, Executive Director of the European Medicines Agency, emphasizes, the value of these connections is profound, often leading to groundbreaking advancements in pharmaceutical R&D. The diverse array of stakeholders attending the DIA 2025 Global Annual Meeting—including regulators, researchers, advocates, and innovators—further enriches the networking landscape, providing R&D managers with unparalleled opportunities to engage and collaborate.
DIA's Influence on Innovation in Pharmaceutical R&D
DIA plays a pivotal role in driving innovation within pharmaceutical R&D by actively promoting the adoption of new technologies and methodologies. This organization not only facilitates the dissemination of research results but also encourages R&D teams to explore advanced solutions that significantly enhance adherence and operational efficiency. The integration of artificial intelligence and machine learning in clinical trials has streamlined data analysis and improved patient recruitment processes. Furthermore, DIA's initiatives support the implementation of digital health technologies, such as wearables and mobile health applications, which provide real-time data and foster greater patient engagement.
In this context, Slickbit's AI-powered Regulatory Intelligence assistant, Lumino, delivers precise, traceable responses from FDA and global guidance documents, bolstering adherence initiatives. Tools like Trend 483 assist in identifying patterns in systemic risks and regulatory issues, while Vault Redact automates the detection and removal of PII and PHI from documents, further empowering R&D teams to navigate the complex regulatory landscape. This unwavering commitment to innovation is essential for organizations striving to maintain a competitive edge in an ever-evolving industry.
Navigating Compliance Challenges with DIA Resources
R&D teams frequently encounter regulatory challenges that can impede their progress in drug development. Notably, over 30% of pharmaceutical R&D professionals report that the integration of data from multiple sources poses a significant challenge. The Drug Information Association (DIA), which is known for its dia full form in medical, offers a comprehensive array of resources, including:
- Detailed guidelines
- Insightful case studies
- Access to expert consultations
All aimed at assisting teams in effectively navigating these hurdles. Furthermore, tools like Slickbit's Trend 483 leverage AI to identify trends in systemic risks and recurring violations from FDA 483s, equipping R&D teams with a deeper understanding of regulatory issues. As highlighted by the Back ONTOFORCE team, "Any compromise in data quality or integrity can lead to erroneous conclusions, wasted resources, and, most critically, risks to patient safety." By utilizing these resources and AI-driven insights, R&D professionals can formulate robust strategies that ensure compliance without sacrificing the integrity of their research. This proactive approach is essential for mitigating risks and achieving successful outcomes in the intricate landscape of drug development. Additionally, maintaining high data quality is crucial, as underscored in the case study on 'Data Quality and Integrity in Pharma R&D,' which emphasizes the importance of efficient data management in ensuring compliance.
Staying Updated: DIA Conferences and Industry Trends
DIA conferences serve as a pivotal platform for R&D professionals, enabling them to stay abreast of the latest industry trends and compliance changes. These events feature expert speakers, panel discussions, and invaluable networking opportunities that yield insights into emerging practices and technologies. By engaging in DIA conferences, R&D teams can secure a competitive edge by understanding the evolving landscape of pharmaceutical advancements and regulatory frameworks.
Furthermore, these gatherings foster collaboration and knowledge sharing, essential for navigating the complexities of the industry. Consequently, participation in DIA conferences is not merely beneficial; it is imperative for those aiming to excel in the pharmaceutical sector.
Future Outlook: DIA's Impact on Pharmaceutical R&D
As the pharmaceutical environment progresses, DIA is strategically positioned to enhance its influence on R&D by adapting to the dynamic shifts in drug development and regulatory compliance. The emergence of new technologies and evolving frameworks underscores DIA's critical role in guiding R&D teams through these complexities. Currently, the enrollment phase accounts for approximately 50% of a clinical trial's duration; thus, DIA's focus on optimizing procedures can significantly reduce overall timelines.
Furthermore, as regulatory scrutiny intensifies, DIA's expertise in navigating compliance challenges becomes increasingly essential. By fostering innovation and collaboration, DIA not only aids pharmaceutical companies in overcoming future obstacles but also ensures they achieve successful outcomes while adhering to evolving standards.
This proactive approach is vital as the industry grapples with pressures from rising healthcare costs and the necessity for efficient drug development strategies, which includes knowledge of the DIA full form in medical.
Conclusion
DIA's pivotal role in enhancing compliance within medical research and drug development is undeniably significant. By acting as a crucial resource for regulatory guidance and collaboration, the Drug Information Association empowers pharmaceutical organizations to navigate the complexities of compliance with increased ease and efficiency. The incorporation of innovative tools and educational initiatives further cements DIA's status as a cornerstone in the pharmaceutical landscape, ensuring that R&D teams are well-equipped to meet evolving regulatory standards.
Key insights throughout the article illustrate how DIA facilitates knowledge exchange, fosters collaboration among industry stakeholders, and provides essential resources for adhering to regulatory requirements. The focus on educational programs, networking opportunities, and the adoption of advanced technologies underscores DIA's commitment to driving innovation and efficiency in pharmaceutical R&D. Moreover, the successful integration of AI solutions, exemplified by Slickbit's tools, showcases how technology can enhance compliance and streamline drug development processes.
In light of these insights, it is imperative for pharmaceutical professionals to actively engage with DIA's resources and initiatives. By doing so, they can not only deepen their understanding of compliance but also contribute to the advancement of the industry as a whole. As the landscape of drug development continues to evolve, proactive involvement in DIA's offerings will be essential in overcoming challenges and ensuring successful outcomes in pharmaceutical research. Embracing this collaborative spirit and leveraging available tools will undoubtedly pave the way for a more efficient and compliant future in life sciences.
Frequently Asked Questions
What is Slickbit and what does it offer for Life Sciences R&D?
Slickbit is a company that provides AI solutions specifically designed for Life Sciences R&D, focusing on rapid MVP development and the integration of AI technologies into drug development processes.
How does Slickbit enhance drug development processes?
Slickbit develops AI agents for regulatory and clinical processes, such as Lumino for regulatory intelligence and Vault Redact for secure content redaction, helping Life Sciences companies navigate complex regulatory environments.
What success rates are associated with AI-driven drug discovery compared to traditional methods?
AI-driven drug discovery has an impressive success rate of 80-90% in Phase I trials, compared to a traditional success rate of 40-65%.
What tools does Slickbit provide to improve operational efficiency?
Slickbit offers tools like Trend 483 for detecting trends in systemic risks and regulatory concerns, an AI Voice Agent for Shipment Alerts, and an AI summarizer to enhance operational efficiency.
What does DIA stand for and what is its significance in the pharmaceutical sector?
DIA stands for Drug Information Association, which plays a crucial role in promoting regulatory adherence and facilitating the exchange of knowledge and best practices within the pharmaceutical sector.
How does DIA assist organizations in navigating regulatory compliance?
DIA provides training and resources that help R&D teams understand and implement essential regulations, enabling them to navigate the complex landscape of drug development compliance.
What is the current trend in regulatory submissions highlighted by DIA?
There is an increasing focus on standardized electronic submissions, particularly the Standard for the Exchange of Nonclinical Data (SEND), which is becoming the preferred format for the U.S. FDA.
How does DIA promote collaboration in pharmaceutical R&D?
DIA enhances collaboration by uniting professionals from academia, industry, and regulatory bodies through workshops, conferences, and networking events, fostering an environment for sharing ideas and best practices.
What are the benefits of DIA's initiatives for drug development?
DIA's initiatives help bridge regulatory bodies and industry participants, ensuring drug development processes align with regulatory standards and enhancing the overall efficiency of drug development.




